Medical Devices: Page 156
-
FDA: Avoid paclitaxel-coated PAD devices for most patients
BD Thursday joined Medtronic in pushing back against the agency's findings, saying it stands behind the safety of the Lutonix drug-coated balloon.
By Susan Kelly • Updated March 22, 2019 -
TAVR called 'game changing' in studies of low-risk patients
Data from Edwards Lifesciences' Partner 3 trial and Medtronic's Evolut Low Risk study prompted predictions that TAVR will become the preferred option for a wider population of heart valve patients.
By Susan Kelly • March 18, 2019 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
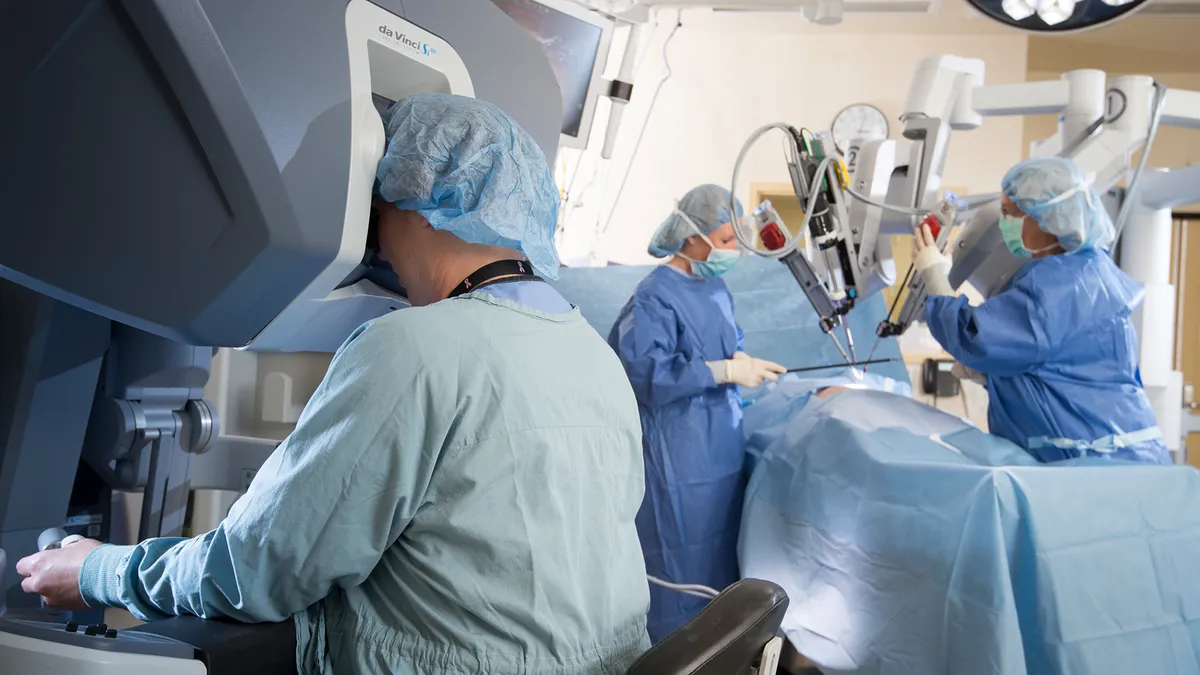
Intuitive's da Vinci SP gets FDA otolaryngology clearance
The approval is the second procedure category to be cleared for the da Vinci single port system, as the company looks to grow internationally.
By Susan Kelly • March 18, 2019 -
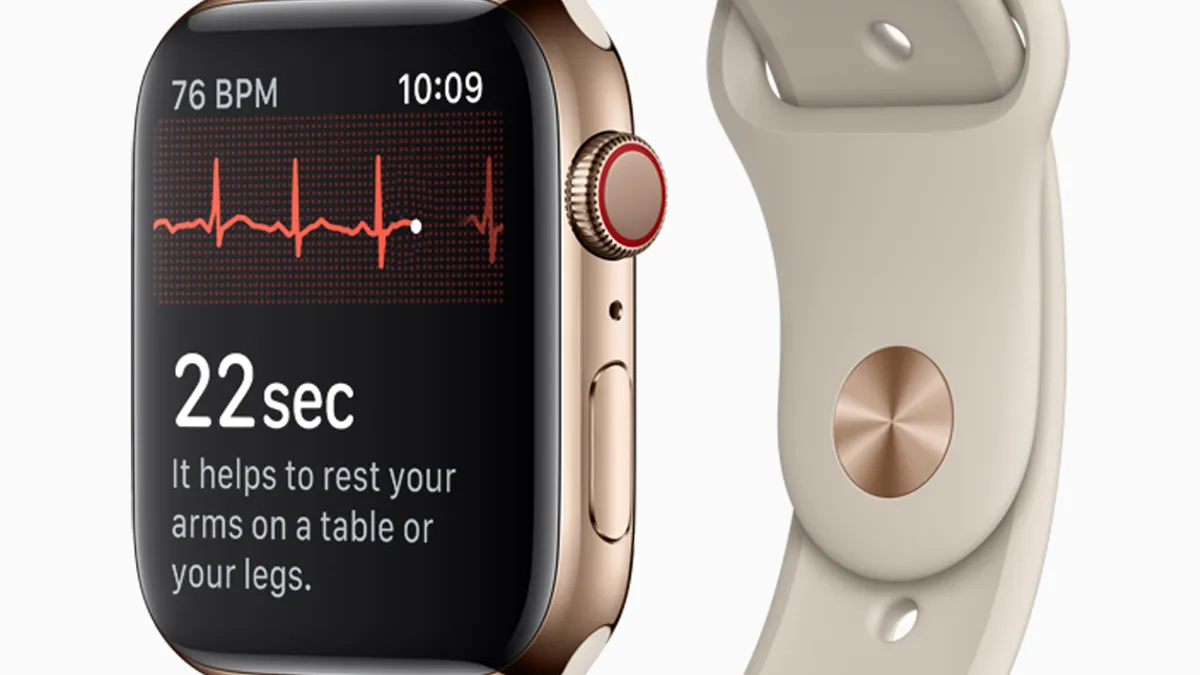
Apple heart study: Early proof of concept, or a gimmick?
Doctors at the American College of Cardiology meeting said the Apple Watch's foray into health monitoring needs more data to prove its value.
By David Lim • March 16, 2019 -
Abbott gains expanded indication for MitraClip valve repair device
M&A has dominated the mitral space in recent years as structural heart competitors look to follow MitraClip's growth.
By Susan Kelly • March 15, 2019 -
FDA greenlights device for treating carbon monoxide exposure
The De Novo-winning Thornhill device accelerates a patient’s breathing rate to get poison out of the body.
By Nick Paul Taylor • March 15, 2019 -
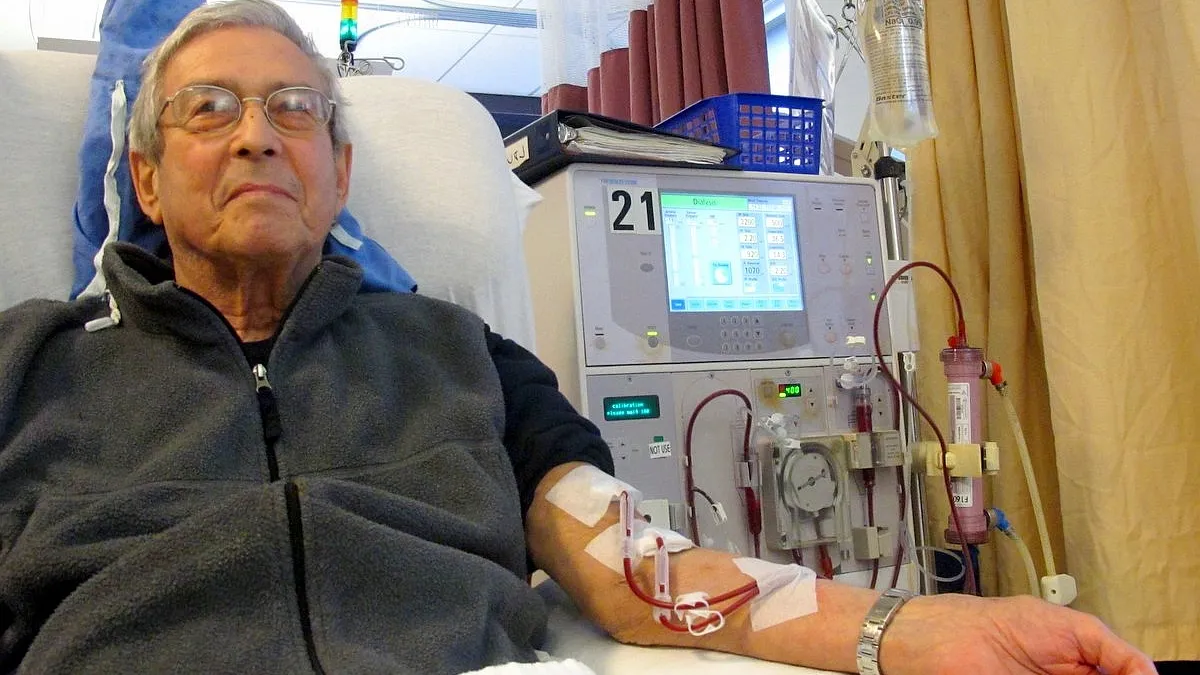
Fresenius gets breakthrough status for hemodialysis software
The experimental software makes recommendations to improve fluid management and thereby cut the rate of complications.
By Nick Paul Taylor • March 15, 2019 -
Apple, Medtronic and more medtech to track at ACC19
Anticipated data backing the tech giant's heart rhythm-tracking watch, results from Medtronic and Edwards aimed at broader use of TAVR procedures, and an update on Abbott's MitraClip highlight the annual conference.
By Maria Rachal • March 14, 2019 -
Stryker to spend up to $220M on rotator cuff tear tech
The Kalamazoo, Michigan-based medtech announced it has closed a deal with Israeli startup OrthoSpace, which formerly drew investment from Johnson & Johnson and Smith & Nephew.
By Maria Rachal • March 14, 2019 -
HemoSonics gets De Novo nod for POC coagulation device
The test uses ultrasound to quickly analyze blood samples at the point of care.
By Nick Paul Taylor • March 14, 2019 -
Navigating FDA's evolving device pathways: A primer
The agency is touting the biggest alterations to medtech approval processes in the decades since it began reviewing devices. Here's a roundup of the current regulatory framework and what's potentially changing.
By Meg Bryant • March 13, 2019 -
FDA qualifies test for traumatic brain injury
The OsiriX CDE test can now be used by medical device developers to identify and enroll patients in studies of TBI, which has been researched for years but has no effective treatment.
By Susan Kelly • March 13, 2019 -
Smith & Nephew buys skin substitute maker for $660M
The company announced Wednesday it closed the acquisition of Osiris Therapeutics.
By Susan Kelly • Updated April 17, 2019 -
Hill-Rom strikes $180M Voalte takeover to boost connected care unit
The acquisition will give the hospital equipment maker a communications system to complement its portfolio of smart devices.
By Nick Paul Taylor • March 13, 2019 -
Omada adds connected blood pressure cuff, glucometer to health program
The devices will enable participants in the digital health platform's disease prevention and management program to collect and share data.
By Nick Paul Taylor • March 13, 2019 -
Diagnostic errors compounded by EHRs No. 1 safety fear: ECRI
"We have to recognize the limits of current technology and ensure that we have processes in place to close the loop on diagnostic tests," the medical device watchdog's William Marcella said.
By Meg Bryant • March 12, 2019 -
NCI Director Ned Sharpless to be named FDA acting commissioner
Sharpless will succeed FDA Commissioner Scott Gottlieb in early April. He has served as director of the National Cancer Institute since October 2017.
By David Lim , Maria Rachal • Updated March 12, 2019 -
Intrathecal drug delivery system wins FDA breakthrough designation
The accelerated review status follows Alcyone's announcement of a strategic collaboration with Roche to develop treatment options for patients with neurological disorders.
By Susan Kelly • March 12, 2019 -
Edwards deepens structural heart position with new investments
Deals with Corvia Medical and Mitralign could bolster the valve specialist's structural heart treatment abilities, where the company said it intends to remain focused.
By Susan Kelly • March 12, 2019 -
FDA digital health efforts highlighted in Trump budget
The blueprint calls for Medicare coverage for breakthrough devices.
By David Lim • March 12, 2019 -
UK to probe safety of paclitaxel cardiovascular devices
The investigation will review data linking drug-coated balloon catheters and drug-eluting stents to increased death rates, including studies of those sold by Boston Scientific, Cook Medical, C.R. Bard, Medtronic and others.
By Nick Paul Taylor • March 12, 2019 -
FDA flags surgical stapler risks, plans advisory meeting
The agency alerted healthcare providers to an increasing number of medical device reports associated with use of surgical staplers, including at least 366 deaths and more than 9,000 serious injuries.
By Susan Kelly • March 11, 2019 -
TAVR for low-risk patients in spotlight at ACC
Jefferies analysts predict results from valve makers Edwards Lifesciences and Medtronic will "usher in the low risk era for TAVR," expanding the market for the devices.
By Susan Kelly • March 11, 2019 -
AdvaMed raises concerns with FDA's proposed De Novo model
In comments on FDA's proposed De Novo classification rule, the medical device lobby said it is concerned the framework would impose some requirements that replicate the premarket approval application review.
By Susan Kelly • March 11, 2019 -
CMS updates 2021 DME bidding round plans, adds new product categories
The American Association for Homecare argued addition of non-invasive ventilators to the competitive bidding program will drive companies out of business.
By David Lim • March 11, 2019