Medical Devices: Page 155
-
CMS greenlights LivaNova's protocol for depression device trial
The U.K. medtech intends to begin enrollment in late 2019, in accordance with the coverage with evidence development framework supporting its vagus nerve stimulation system for use in patients with treatment-resistant depression.
By Susan Kelly • Updated Sept. 6, 2019 -
CMS due to release updated TAVR coverage determination
The agency is reconsidering its existing National Coverage Determination at the urging of physicians who argued that lower-volume heart centers should be allowed to offer the procedure.
By Susan Kelly • March 26, 2019 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
LifeScan dominates Medicare diabetes test strip market, OIG finds
The analysis found LifeScan's OneTouch Ultra held 29% of the non-mail order market, more than twice as much as its nearest rival.
By Nick Paul Taylor • March 26, 2019 -
Biomagnetic imaging for chest pain wins FDA clearance
The rapid, noninvasive imaging test from Genetesis, a Mason, Ohio-based startup, measures the magnetic fields produced by the heart’s electrical activity.
By Susan Kelly • March 25, 2019 -
Not even money prods patients to mail-in colon tests, study suggests
The fecal immunochemical test is used to find small amounts of blood in the stool that could be a sign of cancer or polyps.
By Susan Kelly • March 25, 2019 -
EU device group wants clarity on expert panels under MDR
The coordinating group of the bloc's member states, created to enforce the new device regulations, said the matter is urgent because it is about to launch procedures for establishing the panels.
By Susan Kelly • March 25, 2019 -
With imperfect data, FDA turns to panel for way forward on breast implants
Agency advisers convened Monday for a two-day hearing to discuss how best to characterize and track potential cancer cases linked to the devices, among other issues.
By Maria Rachal • March 22, 2019 -
FDA approves first-of-its-kind heart failure device
The implantable system from Impulse Dynamics is indicated for patients with Class III moderate to severe heart failure who are not eligible for cardiac resynchronization therapy.
By Susan Kelly • March 22, 2019 -
DHS warns of critical cybersecurity weakness with Medtronic implants
The communication system vulnerability, in theory, could enable a hacker with limited skill to change settings on defibrillator implants.
By Nick Paul Taylor • March 22, 2019 -
Key senators demand answers about 'suspect' doctor-device maker ties
Bipartisan leaders of the Senate Finance Committee want CMS to probe whether physician-owned distributorships of medical implants are failing to meet Sunshine Act requirements.
By Nick Paul Taylor • March 22, 2019 -
FDA takes hard look at breast implant safety amid fresh cancer fears
The potential for the devices to cause autoimmune reactions may also be a talking point at Monday and Tuesday's advisory committee meeting. FDA called out potential biocompatibility problems with silicone last week.
By Maria Rachal • March 21, 2019 -
FDA, NEST detail how extra funding can bolster postmarket safety
CDRH Director Jeff Shuren explained the thinking behind the FDA's support of its postmarket surveillance system in a paper with Rachael Fleurence, executive director of the National Evaluation System for health Technology.
By Nick Paul Taylor • March 21, 2019 -
UK steps up no-deal planning as Brexit clock ticks down
MHRA updated its advice on registering devices against a backdrop of concerns about supply disruptions.
By Nick Paul Taylor • March 21, 2019 -
Alzheimer's device De Novo on the line at FDA panel
An advisory committee got started on a transcranial magnetic stimulation device Thursday morning, coming the same day as Biogen halted trials of one of the last late stage drug candidates to treat the disease.
By Susan Kelly • March 20, 2019 -
FDA warns Sientra, J&J's Mentor on lax post-approval breast implant studies
The action comes a week before the agency is set to hold a public meeting on risks related to the controversial devices.
By Susan Kelly • Updated March 20, 2019 -
Sponsored by ZS
Making software a core part of your business model
Pivoting away from devices as the primary revenue stream is proving to be a big challenge for many medtech companies.
By Dan Frey • March 19, 2019 -
BD wins PMA for stent treating venous occlusive disease
The company beat several contenders for the distinction of being first to market in the U.S. with a stent to reopen blocked iliac and femoral veins.
By Susan Kelly • March 19, 2019 -
FDA outlines new device priorities in budget justification report
A new agency document sheds light on proposed initiatives related to cybersecurity, approval pathways and special controls.
By Susan Kelly • March 19, 2019 -
FDA cybersecurity guidance draws fire from big device makers
Boston Scientific, GE Healthcare and BD are among manufacturers raising alarms over a proposal to create two tiers of cybersecurity risk buckets.
By David Lim • March 19, 2019 -
Breast implants, metal hips and other device materials under new FDA scrutiny
Symptoms such as fatigue, rash, joint and muscle pain or weakness may not appear until years after a device is implanted, FDA said.
By David Lim • March 18, 2019 -
JDRF-backed Korean medtech gets breakthrough nod for closed loop system
EOFlow is developing a closed loop automated insulin delivery system for people with Type 1 diabetes as more companies aim to introduce alternatives to Medtronic's 670G MiniMed system.
By Maria Rachal • March 18, 2019 -
FDA: Avoid paclitaxel-coated PAD devices for most patients
BD Thursday joined Medtronic in pushing back against the agency's findings, saying it stands behind the safety of the Lutonix drug-coated balloon.
By Susan Kelly • Updated March 22, 2019 -
TAVR called 'game changing' in studies of low-risk patients
Data from Edwards Lifesciences' Partner 3 trial and Medtronic's Evolut Low Risk study prompted predictions that TAVR will become the preferred option for a wider population of heart valve patients.
By Susan Kelly • March 18, 2019 -
Intuitive's da Vinci SP gets FDA otolaryngology clearance
The approval is the second procedure category to be cleared for the da Vinci single port system, as the company looks to grow internationally.
By Susan Kelly • March 18, 2019 -
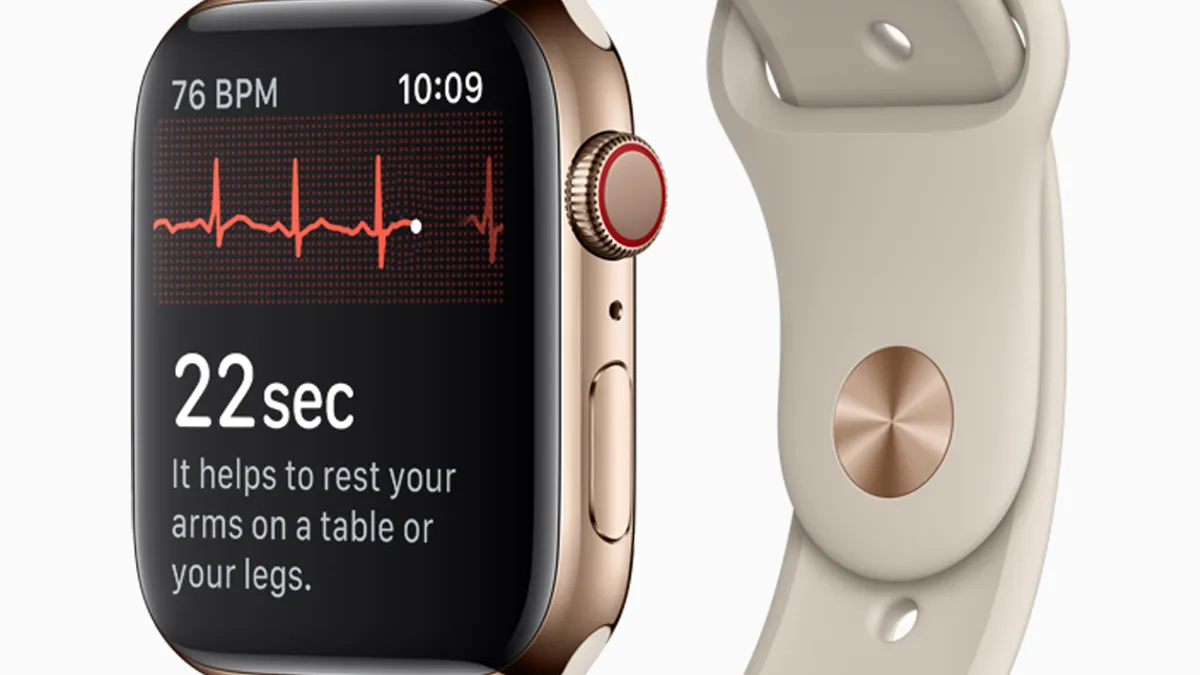
Apple heart study: Early proof of concept, or a gimmick?
Doctors at the American College of Cardiology meeting said the Apple Watch's foray into health monitoring needs more data to prove its value.
By David Lim • March 16, 2019