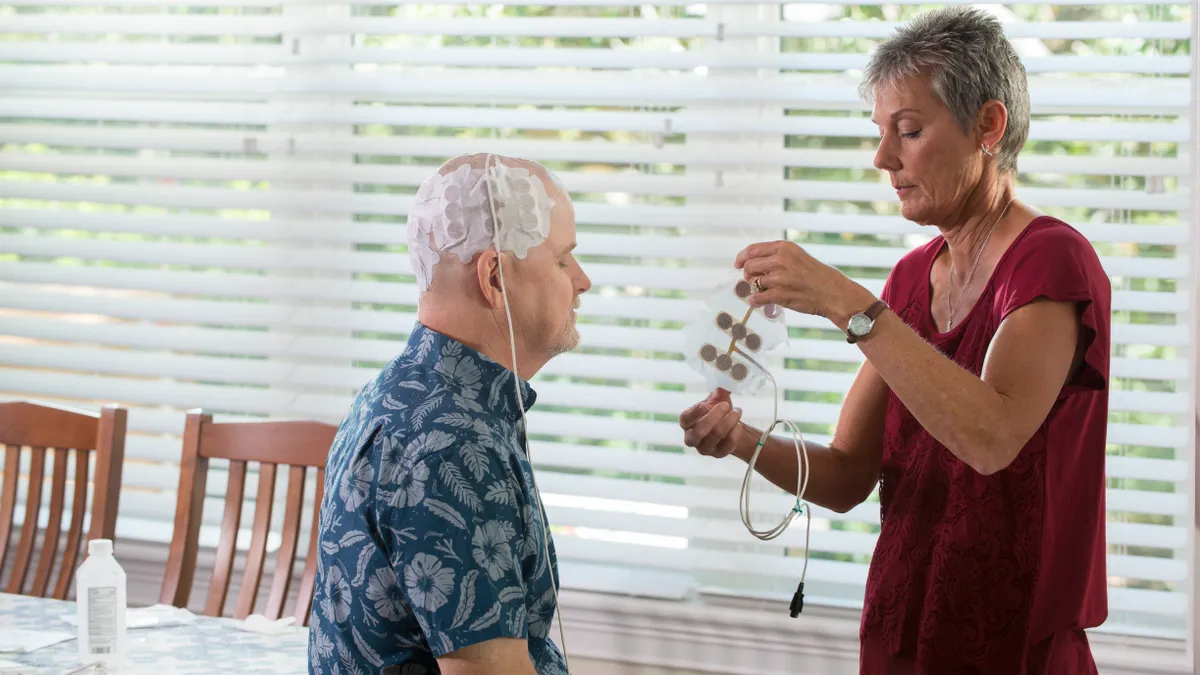Medical Devices: Page 157
-
In bid for new users, Fitbit expands Solera partnership
Fitbit Health Solutions COO Amy McDonough told MedTech Dive many users enrolled in a diabetes prevention program through the partnership choose to later buy a more expensive Fitbit.
By David Lim • March 8, 2019 -
Bard pulls mesh implants from EU
Despite thousands of adverse event reports globally, the BD subsidiary cited the burden of generating clinical data required by new EU rules as the reason for ceasing distribution and sales.
By Nick Paul Taylor • March 8, 2019 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
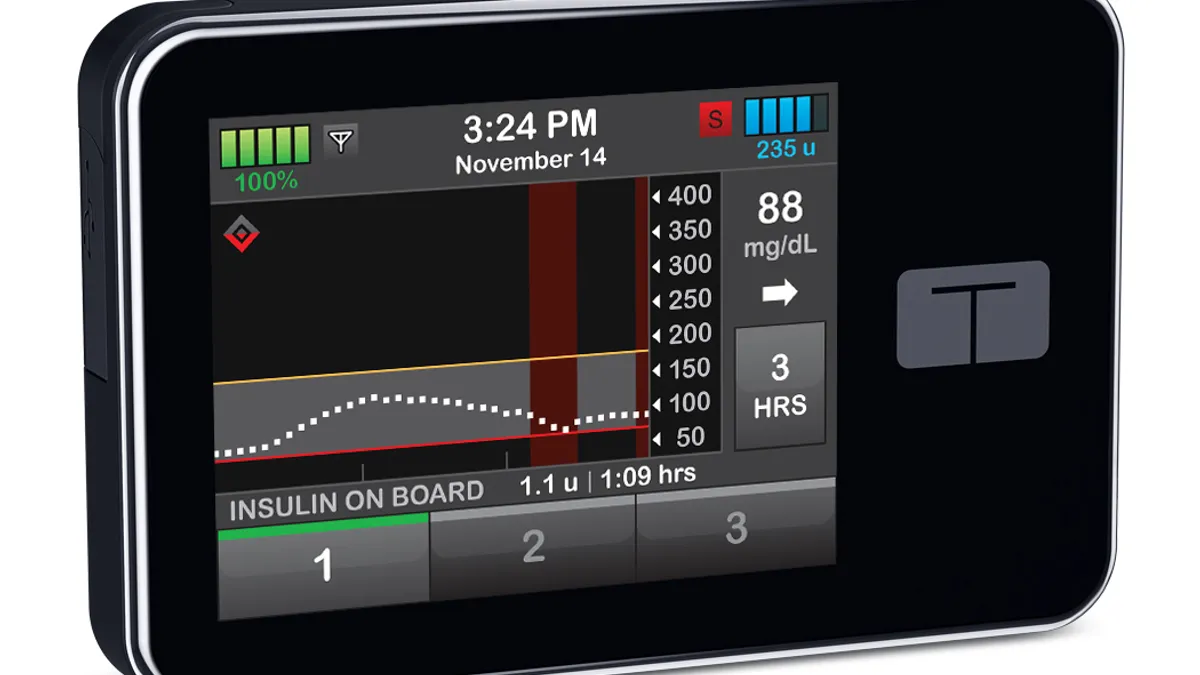
Tandem suspends software use in pivotal closed loop study to fix bug
The company sought to quell nerves, but investors pushed the stock down 8% on safety concerns.
By Nick Paul Taylor • March 7, 2019 -
Medicare panel gives equivocal backing to Novocure's brain cancer device
The panelists expressed some confidence that the Optune device improves outcomes but also raised concerns related to issues like patient compliance.
By Nick Paul Taylor • March 7, 2019 -
Pharma strikes $220M deal for pain device player Myoscience
New Jersey-based Pacira is moving into the device sector to build a portfolio of pain products capable of reducing the need for opioids.
By Nick Paul Taylor • March 6, 2019 -
FDA proposes exempting some flow cytometers from 510(k) requirements
The change is intended to streamline the path to market for some devices that count or characterize cells.
By Nick Paul Taylor • March 6, 2019 -
Device to detect internal bleeding wins De Novo clearance
The monitoring system targets patients who could experience complications during percutaneous procedures using large diameter catheters such as TAVR.
By Susan Kelly • March 5, 2019 -
Masimo brain monitor gets CE mark pediatric indication
The device is now available for use in patients between ages 1 and 18 who undergo anesthesia for surgical or diagnostic procedures.
By Susan Kelly • March 5, 2019 -
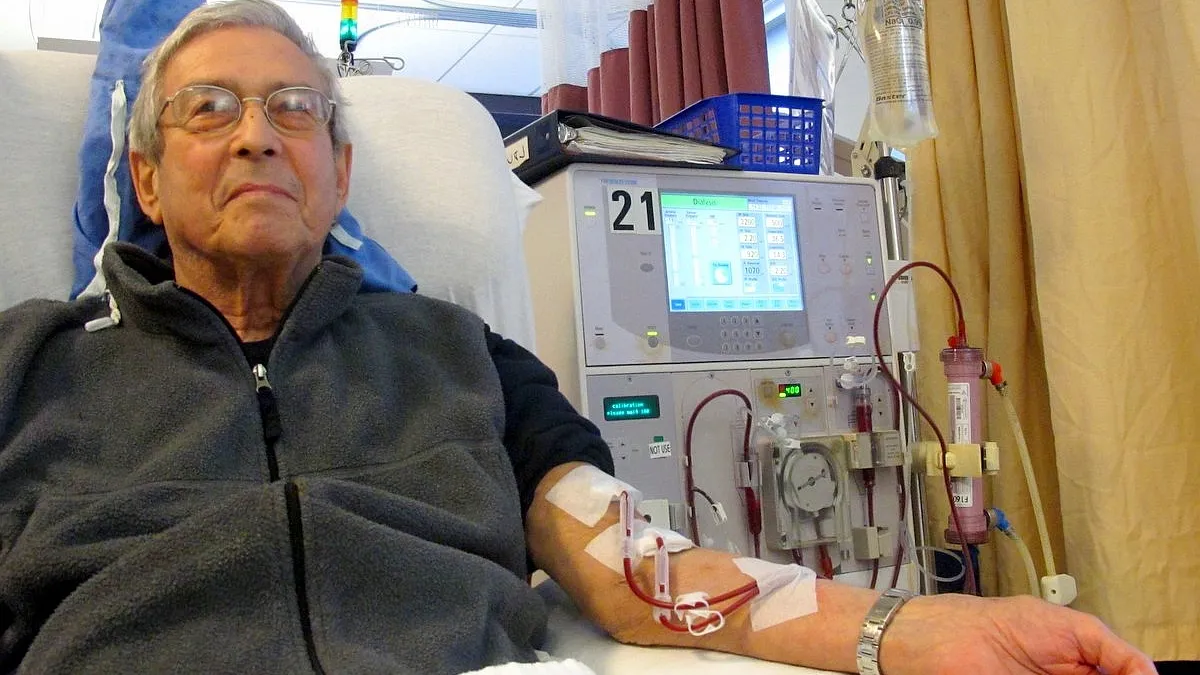
US kidney care getting revamp, HHS officials say
HHS Secretary Alex Azar pushed for the development of wearable and implantable artificial kidneys.
By Rebecca Pifer • March 4, 2019 -
Deep Dive
Mixing medicine and money: Why the rise of health system VCs is raising ethical concerns
More dollars than ever are flowing from VCs affiliated with nonprofit health systems, spurring questions around conflicts of interest.
By Taylor McKnight • March 4, 2019 -
CVS to close retail audiology centers ahead of over-the-counter regulations
FDA has made clear it believes consumers should be able to take a more active role in managing hearing loss.
By David Lim • March 4, 2019 -
Medtronic says corrected IN.PACT analysis to be published in JACC
The revision, prompted by a data error, complicates an already contentious debate about long-term safety of drug-coated balloons in treating peripheral artery disease.
By Susan Kelly • March 4, 2019 -
Scientists propose universal standard to calibrate CT scanners
The measurement approach could improve comparison of scans from different machines, potentially making diagnosis more efficient and less costly.
By Susan Kelly • March 4, 2019 -
As robotic surgery booms, FDA cautions against use for cancer
Agency leaders cited lack of evidence on safety and effectiveness, but major health systems report using the tech for cancer patients.
By Maria Rachal • March 1, 2019 -
Gene test for antibiotic resistance gets FDA breakthrough nod
The World Health Organization has identified antibiotic resistance as one of the biggest threats to global health.
By Susan Kelly • March 1, 2019 -
EU debuts drug-device Q&A ahead of regulatory changes
The guidance sets out how incoming medical device regulations will affect developers of pre-filled syringes and other combination products.
By Nick Paul Taylor • March 1, 2019 -
Moody's warns medtech is highly vulnerable to cyberattacks
The credit rating agency said impact of an attack would still not rival the severity of one on the broader healthcare industry.
By Nick Paul Taylor • March 1, 2019 -
Gottlieb pledges device, drug reviews will be on time post shutdown
The FDA chief's appearance before Congress comes a day after a bill was introduced to allow the agency to accept new medical device applications and user fees during a government shutdown.
By David Lim • Feb. 28, 2019 -
Boston Scientific Lotus matches Medtronic CoreValve in head-to-head TAVR trial
The readout comes as Boston Scientific prepares to introduce its device in the U.S. and Europe.
By Nick Paul Taylor • Feb. 28, 2019 -
Hologic gets EU clearance for breast lesion localization tag
The device uses radio frequency identification to wirelessly guide surgeons to breast lesions.
By Nick Paul Taylor • Feb. 28, 2019 -
UK updates Brexit medtech advice as cliff edge exit looms
The Medicines and Healthcare products Regulatory Agency document sheds new light on the responsible person requirement and parallel importing policy.
By Nick Paul Taylor • Feb. 28, 2019 -
Medtronic Resolute stent gains expanded indication
The device will now compete against Abbott's Xience for patients with a complex form of coronary artery disease known as chronic total occlusion.
By Susan Kelly • Feb. 27, 2019 -
Thermo Fisher lung cancer test wins expanded approval in Japan
Japan's regulatory agency approved the Oncomine DX Target test to include three additional biomarkers for non-small cell lung cancer.
By Susan Kelly • Feb. 27, 2019 -
Medtronic ablation offerings heat up with Accurian launch
The medtech said Wednesday it received 510(k) FDA clearance and has begun the U.S. launch of a nerve ablation system targeting chronic pain.
By Maria Rachal • Feb. 27, 2019 -
Introduction of robotic surgery cuts endometrial cancer complications: JAMA
The study linked minimally invasive procedures to significantly fewer severe complications than open procedures.
By Nick Paul Taylor • Feb. 27, 2019