FDA: Page 5
-
Deep Dive
A judge blocked the FDA’s plan to regulate LDTs. What now?
One attorney said the ruling “isn’t quite the stake in the heart.”
By Susan Kelly • April 16, 2025 -

 Retrieved from Intuitive Surgical on April 11, 2025
Retrieved from Intuitive Surgical on April 11, 2025
Intuitive wins FDA clearance for single-port robotic surgery stapler
CFO Jamie Samath said in January that the stapler clearance would trigger the start of “broad commercial efforts” for the single-port system.
By Nick Paul Taylor • April 11, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
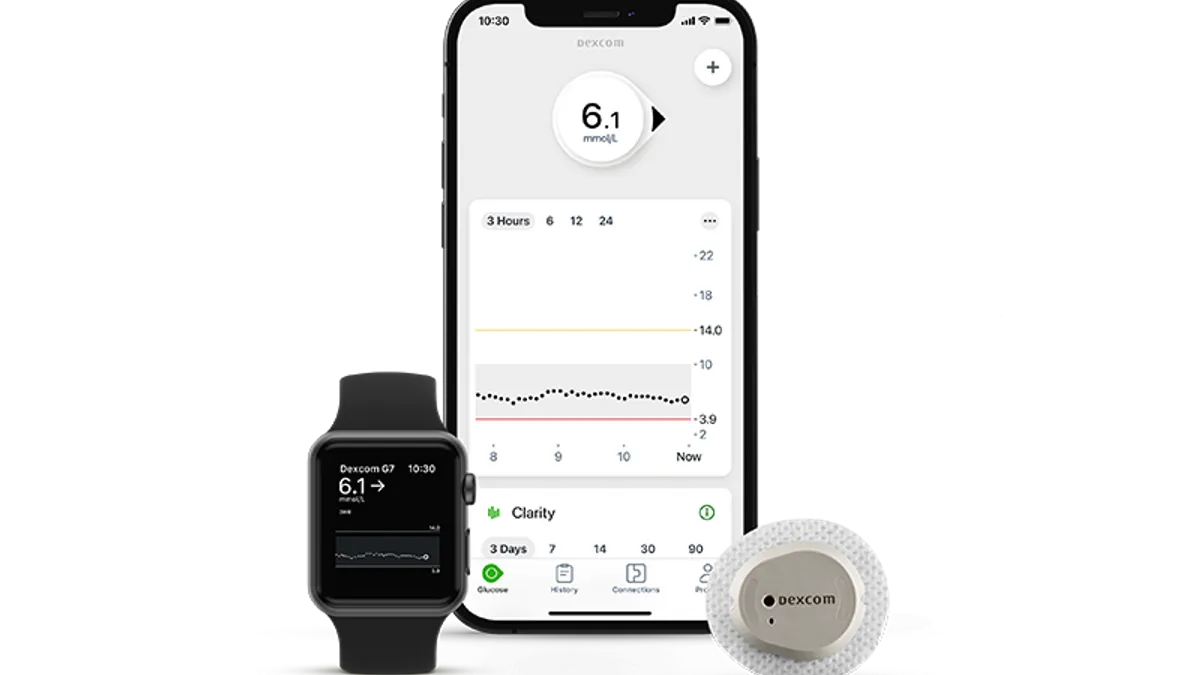
Dexcom nets FDA clearance for 15-day CGM
The longer wear time should improve Dexcom’s margins and help it compete with Abbott, RBC Capital Markets analyst Shagun Singh said.
By Elise Reuter • April 10, 2025 -
RFK Jr. won’t appear before Senate panel this week
HELP committee chair Bill Cassidy, R-La., had invited Kennedy to testify April 10 on the sweeping layoffs of federal health workers Kennedy ordered last month. A hearing could still take place later.
By Ned Pagliarulo • April 8, 2025 -
HHS overhaul
HHS layoffs characterized by confusion, errors
The sweeping workforce reduction that began Tuesday was made worse by mistakes and poor communication, including directions for some CMS employees to contact a director who died last year.
By Rebecca Pifer • Updated April 2, 2025 -
HHS overhaul
HHS layoffs could imperil medical device cybersecurity, Democrats say
The FDA has 3,500 jobs on the chopping block, which could hinder cybersecurity oversight, Democrats said during a House subcommittee hearing.
By Emily Olsen • April 2, 2025 -
HHS overhaul
‘Just goodbyes and crying’: CDRH hit in HHS mass layoffs
Teams working on communications and policy were cut from the agency on April 1, according to multiple FDA workers interviewed by MedTech Dive.
By Elise Reuter • April 2, 2025 -
HHS overhaul
HHS begins layoffs in chaotic fashion
The Trump administration sent out the first round of reduction-in-force notices early Tuesday morning, telling employees the cuts are necessary to improve efficiency.
By Rebecca Pifer • April 1, 2025 -
Texas judge overturns FDA’s lab developed test regulation, siding with industry groups
The judge vacated the FDA’s final rule, which was strongly opposed by the laboratory industry, and remanded the matter to HHS Secretary Robert F. Kennedy Jr.
By Susan Kelly • April 1, 2025 -

 Ermath, Michael. (2020). "Individualized Therapies Workshop" [Photograph]. Retrieved from Flickr.
Ermath, Michael. (2020). "Individualized Therapies Workshop" [Photograph]. Retrieved from Flickr.
Peter Marks, FDA’s top vaccine official, resigns
In his resignation letter, Marks cited disagreement with HHS Secretary Robert F. Kennedy Jr., who he said pushed “misinformation and lies.”
By Ned Pagliarulo • March 31, 2025 -
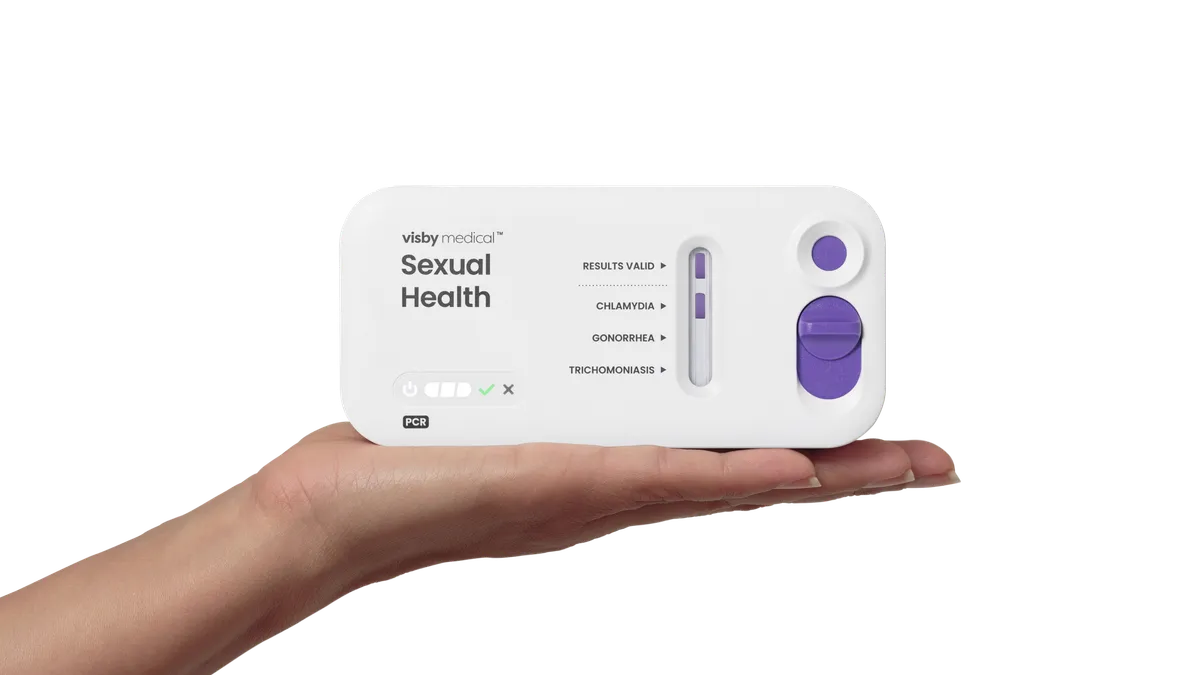
FDA OKs first at-home test for 3 STIs
Visby Medical’s at-home diagnostic, which can be purchased without a prescription, tests for chlamydia, gonorrhea and trichomoniasis.
By Nick Paul Taylor • March 31, 2025 -
HHS overhaul
‘Everything is word of mouth’: HHS employees face uncertainty in looming Trump layoffs
Many HHS department heads and employees were unaware of plans to fire some 10,000 employees until they were announced Thursday, sources said.
By Sydney Halleman , Ned Pagliarulo , Rebecca Pifer • March 28, 2025 -
Dexcom rejects claims of unauthorized device changes in FDA warning letter
A company spokesperson said Dexcom plans to resolve the agency's concerns but stated no design changes were made to its glucose sensors.
By Elise Reuter • March 27, 2025 -
HHS overhaul
HHS to cut 10,000 employees in major restructuring under RFK Jr.
Advamed CEO Scott Whitaker said Thursday that “any reduction in force should be accompanied by policy and regulatory improvements that encourage innovation in medtech.”
By Ned Pagliarulo , Rebecca Pifer , Ricky Zipp • Updated March 27, 2025 -
Makary confirmed by Senate as FDA commissioner
The Johns Hopkins surgeon will helm the agency as layoffs loom and important decisions on treatments for rare and infectious diseases await.
By Jonathan Gardner • March 26, 2025 -
Dexcom’s FDA warning letter reveals unauthorized changes to sensors
Dexcom made a significant design change to a component used in its sensors and did not adequately validate the change, according to the warning letter.
By Elise Reuter • March 26, 2025 -
Deep Dive
5 tips for building a predetermined change control plan
The new framework is intended to make postmarket changes easier for products. Experts recommend having a clear roadmap and contacting regulators early.
By Elise Reuter • March 25, 2025 -
Abbott gets FDA nod to begin IVL trial after rivals buy up competition
J&J acquired the IVL device maker Shockwave Medical last year for $13.1 billion, and Boston Scientific agreed to buy Bolt Medical in January for up to $664 million.
By Nick Paul Taylor • March 25, 2025 -

 Retrieved from ICU Medical on March 21, 2025
Retrieved from ICU Medical on March 21, 2025
Smiths Medical recalls port implants, warns on endotracheal tubes
The business, acquired by ICU Medical in 2022, has been dealing with a series of recalls and quality issues over the past several years.
By Susan Kelly • March 21, 2025 -
FDA posts early alert after Calyxo urinary stone device tied to death
The alert describes a problem that can lead to excessive pressure in the kidney during procedures to remove urinary stones.
By Nick Paul Taylor • March 21, 2025 -
Medtronic recalls embolization devices tied to 17 injuries, 4 deaths
Medtronic will remove one model of Pipeline Vantage devices from where they’re used and sold and will update the instructions for another model.
By Nick Paul Taylor • March 19, 2025 -
Monogram robot wins FDA OK; Vicarious hit by supplier woes
Monogram Technologies secured 510(k) clearance for its robotic knee replacement system, while Vicarious Surgical blamed supply chain issues for delaying its regulatory timeline.
By Susan Kelly • March 19, 2025 -
Top device firms report safety data late: BMJ
BD, Medtronic and Abbott were among the top 10 device companies with the highest number of late reports to the FDA, the study found.
By Elise Reuter • March 18, 2025 -
FDA adds hemodialysis devices to shortage list amid B. Braun disruption
The disruption is expected to impact patient care, may require adjustments to the management of hemodialysis patients and could continue through the early fall of 2025.
By Nick Paul Taylor • March 17, 2025 -

 Retrieved from Screenshot: Johnson & Johnson on March 13, 2025
Retrieved from Screenshot: Johnson & Johnson on March 13, 2025
J&J’s Monarch robot wins FDA nod for AI software update
Using Nvidia and GE Healthcare technology, the robotic surgery system is intended to help doctors detect lung cancer earlier.
By Susan Kelly • March 13, 2025