FDA: Page 4
-
Q&A
CDRH cyber official on compliance with new rules, ongoing security threats
Nastassia Tamari discussed compliance with new device cybersecurity requirements, the risks of legacy devices and frequent cyberattacks in healthcare.
By Ricky Zipp • Updated Dec. 17, 2024 -
4 takeaways from the FDA’s first digital health advisory committee
Industry and patient representatives debated how the FDA should regulate generative AI in medical devices and address new challenges with the technology, such as the use of complex models that can change quickly.
By Elise Reuter • Dec. 12, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
How Trump’s second term will affect the medtech industry
From M&A and tariffs to new health leaders, here are the top issues for the medtech industry to watch during President Donald Trump’s return to office.
By Ricky Zipp • Dec. 10, 2024 -
FDA issues final guidance on postmarket updates to AI-enabled devices
By using pre-determined change control plans, manufacturers can update AI-enabled devices without filing an additional FDA submission.
By Elise Reuter • Dec. 4, 2024 -
Movano receives FDA nod for smart ring’s pulse oximeter feature
Movano Health plans to market the Evie Ring to organizations that run clinical trials and healthcare companies that are helping patients manage chronic diseases.
By Nick Paul Taylor • Dec. 3, 2024 -
Advamed CEO stresses need for safety, efficacy after Trump picks Makary to lead FDA
Martin Makary, a Johns Hopkins surgeon and researcher, has been critical of the FDA. He could have the most influence over device policy among Trump’s nominees.
By Ricky Zipp • Nov. 27, 2024 -
Zimmer wins FDA approval for cementless partial knee implant
The device is established in Europe, where the company said it has a 60% market share, but will be the first product of its type available in the U.S.
By Nick Paul Taylor • Nov. 26, 2024 -
Johns Hopkins surgeon Makary is Trump’s pick to lead FDA
A prolific medical researcher and author, Martin Makary criticized the FDA and CDC for their decision-making during the pandemic, although he describes himself as pro-vaccine.
By Jonathan Gardner , Ned Pagliarulo • Nov. 24, 2024 -
Medtronic nets FDA nod for smart insulin pen app
The clearance paves the way for the launch of Medtronic’s Smart MDI system, which combines its InPen and Simplera continuous glucose monitor for people who take multiple daily insulin injections.
By Susan Kelly • Nov. 22, 2024 -
FDA plan would alert public sooner on high-risk device recalls
The Center for Devices and Radiological Health’s pilot program is meant to cut the time between the FDA’s awareness of potentially high-risk recalls and when the public is notified.
By Ricky Zipp • Nov. 22, 2024 -
Healthcare industry braces for RFK Jr.
Kennedy's views on vaccines in particular are causing alarm among some physicians and investors.
By Susanna Vogel • Nov. 22, 2024 -
Philips revises ventilator directions after airflow issue tied to 4 injuries
Aerosol deposits could accumulate on a sensor and affect oxygen delivery, the FDA said in a recall notice. Several of the ventilator models were previously recalled for separate problems.
By Susan Kelly • Nov. 21, 2024 -
FDA warns providers on Getinge devices tied to 17 serious injuries
Getinge recalled endoscopic vessel harvesting devices after receiving 18 complaints in four months. The FDA added the devices to its shortages list following the recall.
By Nick Paul Taylor • Nov. 18, 2024 -
Trump nominates RFK Jr. to lead HHS
The nomination of a prominent vaccine skeptic to the head of the nation’s largest healthcare program is expected to alarm some public health experts.
By Sydney Halleman • Nov. 14, 2024 -
FDA breakthrough device decisions rebound after recent declines
The FDA designated 166 products under its breakthrough devices program in its most recent financial year, reversing the downward trend seen as the COVID-19 pandemic eased.
By Nick Paul Taylor • Nov. 14, 2024 -
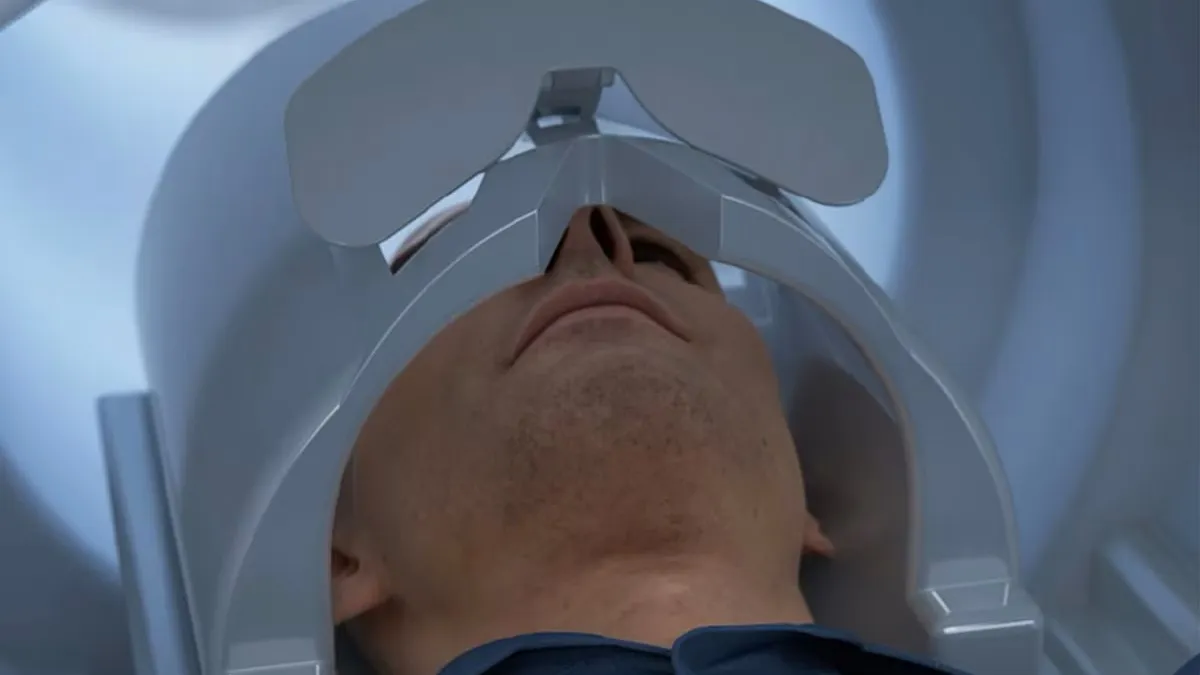
 Retrieved from GE Healthcare on November 14, 2024
Retrieved from GE Healthcare on November 14, 2024
GE Healthcare’s head-only MRI scanner cleared by the FDA
The system is designed to shorten scan times, which may be better for people who struggle to stay still or have claustrophobia, and to detect more subtle abnormalities.
By Nick Paul Taylor • Nov. 14, 2024 -
Advamed CEO congratulates Trump, stresses need for public policy support
Donald Trump’s election victory comes weeks after the FDA named a new leader for the medical device center and as the agency’s contentious LDT final rule is challenged in court.
By Ricky Zipp • Nov. 11, 2024 -
How the healthcare industry is reacting to a second Trump term
Donald Trump’s first term as president was characterized by significant turbulence for government healthcare programs. Here’s how some of the most influential industry groups responded to the Republican’s reelection.
By Rebecca Pifer • Nov. 11, 2024 -

 Retrieved from Aerial Lens on October 10, 2024
Retrieved from Aerial Lens on October 10, 2024
Baxter to restart second IV fluid production line at hurricane-damaged site
CFO Joel Grade told investors Friday that Baxter expects an approximately $200 million hit to sales in the fourth quarter from Hurricane Helene's disruption.
By Susan Kelly • Nov. 11, 2024 -
J&J wins FDA approval for Varipulse PFA system
J&J is the third medtech company to gain U.S. approval for pulsed field ablation, a new atrial fibrillation treatment seeing rapid adoption.
By Susan Kelly • Nov. 7, 2024 -
With Trump victorious, biotech industry’s focus turns to his plans for FDA, FTC
The president-elect has said he’ll let Robert F. Kennedy Jr. “go wild” on healthcare, while many expect a leadership change at the FTC could lower M&A scrutiny.
By Ned Pagliarulo , Ben Fidler • Nov. 7, 2024 -
Advamed asks CMS to cover extra imaging of dense breast tissue
Many patients with dense breast tissue currently have to pay out of pocket or forgo potentially life-saving additional testing, Advamed said in a letter.
By Nick Paul Taylor • Nov. 6, 2024 -
Medtronic settles pulse oximetry lawsuit alleging inaccuracies
Roots Community Health has sued 12 other firms, including GE Healthcare, CVS and Walgreens, and called for manufacturers and the FDA to ensure the devices work for everyone.
By Elise Reuter • Nov. 4, 2024 -

 Retrieved from iRhythm on October 22, 2024
Retrieved from iRhythm on October 22, 2024
FDA clears iRhythm’s second 510(k) in response to warning letter
However, the company said it will delay a submission for its next-generation Zio cardiac monitoring system until remediation efforts are further along.
By Susan Kelly • Nov. 1, 2024 -
Michelle Tarver faces challenges as new CDRH leader. But patient groups, industry are optimistic.
Patient advocates are hoping for change under new director Michelle Tarver, while industry groups look to build on former director Jeff Shuren’s leadership.
By Elise Reuter • Oct. 31, 2024