FDA: Page 32
-
Puerto Rico-based lab ceases COVID-19 test; FDA revokes EUA
The FDA disclosed the action alongside updates to the instructions for use for Thermo Fisher Scientific’s TaqPath COVID-19 test.
By Nick Paul Taylor • Sept. 26, 2022 -
Philips’ latest BiPAP machine recall labeled Class I event by FDA
Certain bi-level positive airway pressure devices feature plastic that could cause machines to stop working suddenly, potentially leading to serious injury or death, the FDA said Friday.
By Nick Paul Taylor • Sept. 26, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Device makers with ethylene oxide facilities at risk of lawsuits after Sterigenics loss: Needham
The $363 million award in the Sterigenics lawsuit “could lead to increased aggressiveness on the part of attorneys,” Needham analysts warned in a Friday note to investors.
By Ricky Zipp • Sept. 23, 2022 -
FDA user fee package to be included in Congress’ bill to fund government, avoid shutdown, Senators say
A “practically clean” version of the bill will allow the FDA to continue its review and testing programs, while leaving more ambitious goals to a funding bill later this year.
By Elise Reuter • Sept. 22, 2022 -
As deadline for FDA user fees bill nears, Congress weighs continuing resolution
Reauthorization bill is likely to pass before Sept. 30, but it’s unclear what provisions will be included in the final version, experts said.
By Elise Reuter • Updated Sept. 22, 2022 -
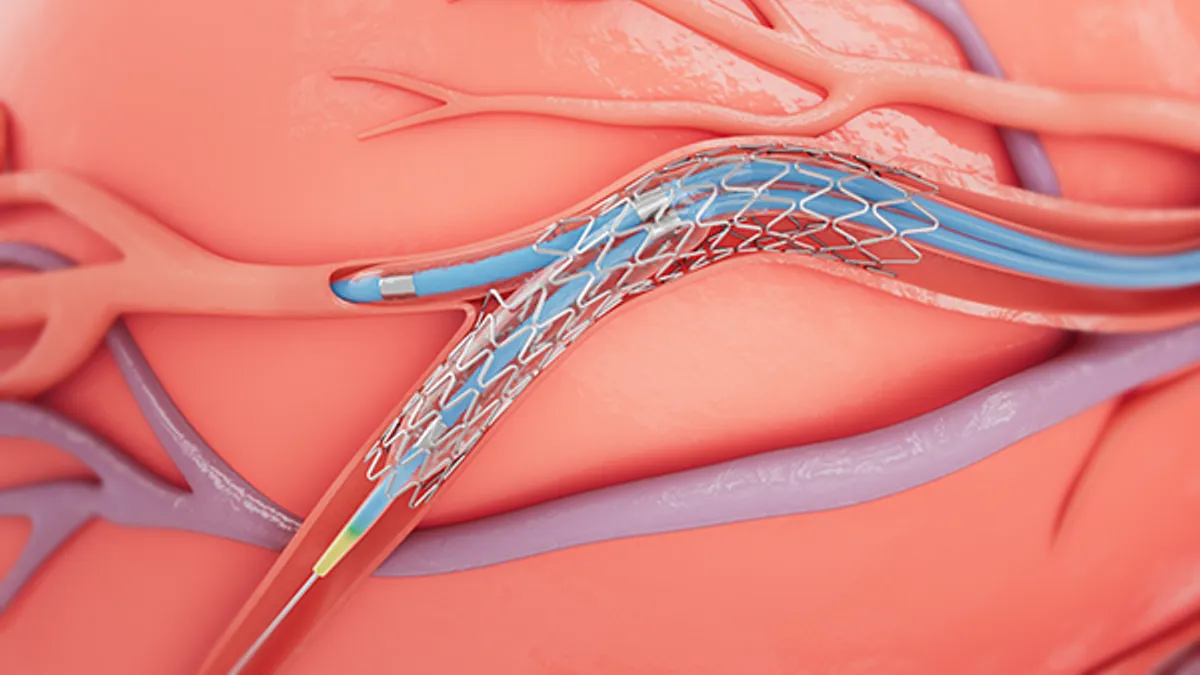
 Courtesy of https://filecache.mediaroom.com/mr5mr_medtronic/183509/SURTAVI%20%28002%29%209.21%20600pix.png
Courtesy of https://filecache.mediaroom.com/mr5mr_medtronic/183509/SURTAVI%20%28002%29%209.21%20600pix.png
Medtronic gets first FDA approval for drug-eluting stent in bifurcation lesions
Researchers have found the one-device, provisional stenting method delivers better results than two stents in some patients.
By Nick Paul Taylor • Sept. 22, 2022 -
Watchdog faults FDA for rushing COVID-19 tests to market by easing emergency use rules
HHS’ Office of Inspector General found that by loosening emergency use authorization requirements to bring COVID-19 tests to market faster, the agency allowed inaccurate tests to be distributed.
By Elise Reuter • Sept. 21, 2022 -
Insulet’s Omnipod 5 gains CE Mark for European release
After months of regulatory delays, Insulet’s newest insulin pump can now be marketed in the U.S. and European Union.
By Nick Paul Taylor • Sept. 21, 2022 -
Jury decides against Sterigenics in ethylene oxide trial, awarding $363M to the sole plaintiff
Sterigenics has more than 760 lawsuits pending against it in the local court for toxic emissions previously emitted by the plant.
By Nick Paul Taylor • Sept. 20, 2022 -
FDA updates advice about Medtronic endotracheal tubes after Class I recall, patient death reports
The FDA provided more detailed recommendations on preventing and responding to airway obstruction that may occur when using some Medtronic endotracheal tubes.
By Peter Green • Sept. 19, 2022 -
Racial bias in pulse oximeters to be subject of FDA panel
The design of the ubiquitous fingertip monitors has inadvertently caused false blood oxygen readings in darker-skinned patients, a problem the FDA hopes to fix.
By Peter Green • Sept. 16, 2022 -
Baxter’s recall of solution sets used in chemotherapy labeled Class I by FDA amid complaints of leaks
The regulator says the leaks could kill, but with 83 complaints it has not received reports of patient injuries or deaths.
By Ricky Zipp • Sept. 15, 2022 -
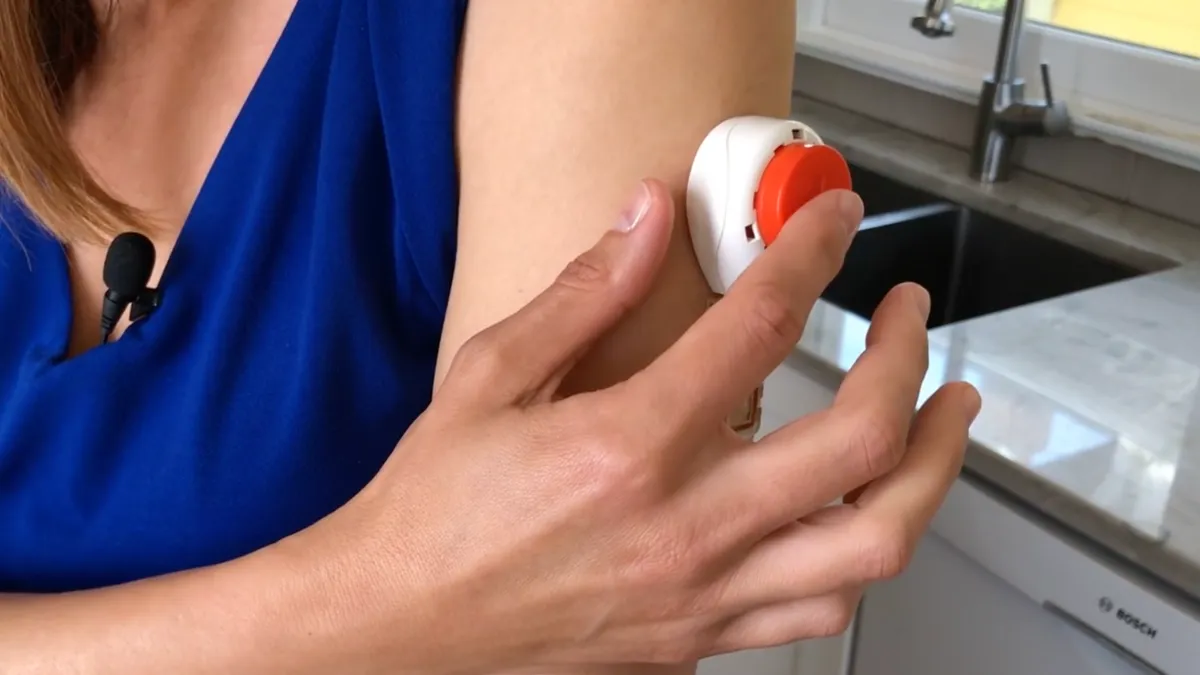
 Retrieved from https://www.tassoinc.com/product-use-videos on September 15, 2022
Retrieved from https://www.tassoinc.com/product-use-videos on September 15, 2022
Tasso’s at-home blood collection lancet wins FDA clearance
Tasso is pitching the device as an enabler of decentralized clinical trials because it could simplify the collection of blood at home.
By Nick Paul Taylor • Sept. 15, 2022 -
Edwards updates Sapien TAVR device for greater durability
The device uses Edwards’ Resilia tissue technology to enable dry storage and potentially extend device durability.
By Nick Paul Taylor • Sept. 14, 2022 -
Investor lawsuit accuses Medtronic of failing to disclose insulin pump problems
The plaintiffs claim Medtronic failed to disclose that its product quality control systems were inadequate and that it was noncompliant with regulations.
By Nick Paul Taylor • Sept. 13, 2022 -
J&J agrees to settle class action pelvic mesh suits in Australia for more than $200M
Shine Lawyers, the firm representing the patients, said that the Federal Court of Australia still needs to approve the terms of the agreement.
By Ricky Zipp • Sept. 13, 2022 -
Philips says French prosecutors investigating respirator recall
While the company confirmed it faces lawsuits in France, Philips said it’s too early “to speculate about any potential exposure.”
By Elise Reuter • Sept. 9, 2022 -
FDA alerts physicians to lock malfunction with Abbott’s MitraClip heart valve device
The problem, which the FDA said occurs in 1.3% of procedures, can have consequences including the need for surgery to treat significant residual disease.
By Nick Paul Taylor • Sept. 9, 2022 -
FDA: Reports of squamous cell carcinoma, lymphomas in scar tissue around breast implants
The report adds to the history of product safety problems for breast implant devices, including patient injuries and deaths.
By Ricky Zipp • Sept. 8, 2022 -
French prosecutors investigating Philips Respironics recall: Reuters
In addition to a U.S. DOJ inquiry, Philips now faces an investigation from French prosecutors over its recall of sleep apnea machines and ventilators.
By Elise Reuter • Sept. 8, 2022 -
FDA issues first EUA for monkeypox test
Quest Diagnostics' PCR test for monkeypox received emergency use authorization after the FDA issued guidance on how it will evaluate the tests.
By Elise Reuter • Sept. 8, 2022 -
HHS will allow EUAs for monkeypox tests to increase access in the US
The move follows the declaration of a public health emergency for the monkeypox outbreak on Aug. 4.
By Ricky Zipp • Sept. 7, 2022 -
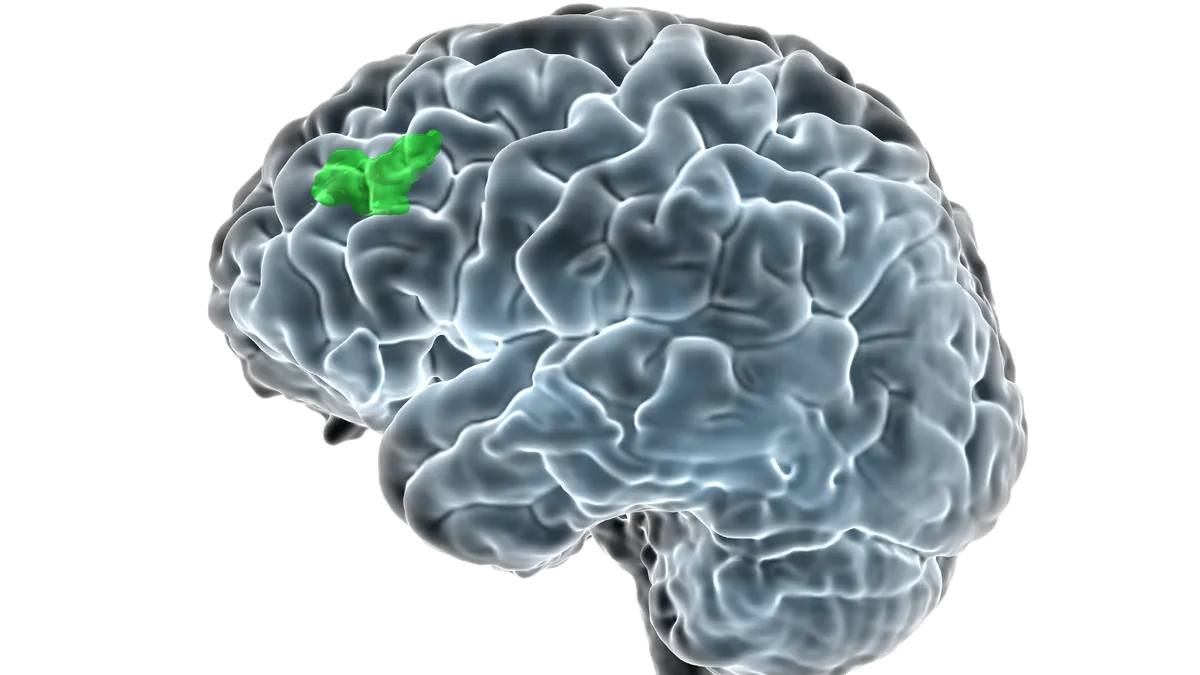
FDA clears Magnus neuromodulation system in major depressive disorder
Almost 80% of people treated with the SAINT system entered remission, compared to 13% of their peers in the control group.
By Nick Paul Taylor • Sept. 7, 2022 -
Philips recalls 17M sleep apnea masks over magnets that could affect implanted devices
The problem has caused 14 serious injuries, and the FDA cautioned that the issue could lead to deaths.
By Nick Paul Taylor • Updated Sept. 8, 2022 -
Illumina’s hold on Grail depends now on separate appeals in US, Europe
Even as it plans to appeal a European Commission decision and the FTC plans to appeal a U.S. ruling, Illumina say it’s preparing for ‘strategic alternatives’ to its acquisition of early cancer detection firm Grail.
By Elise Reuter • Updated Sept. 7, 2022