FDA: Page 31
-
European Commission targets spring of 2024 for fully functional Eudamed database
The Commission is planning a two-phase transition to mandatory use of the database after it goes live.
By Nick Paul Taylor • July 11, 2022 -
Medtronic recalls over 1M dialysis catheters due to malfunction
The recall is the latest in a growing list of product safety issues for Medtronic, including Class I recalls and an FDA warning letter for its diabetes unit.
By Ricky Zipp • July 11, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Ex-Mazor executive charged by SEC with insider trading over Medtronic's $1.6B buyout
Doron Tavlin, who was Mazor’s vice president of business development, is accused by the agency of profiting from his inside knowledge of the pending takeover.
By Nick Paul Taylor • July 11, 2022 -
CMS proposes national rates for cardiac monitoring; iRhythm's stock jumps
The proposed rule could end a years-long saga for the cardiac market, although pricing specifics may change in the final version.
By Ricky Zipp • July 8, 2022 -
High-risk IVD tests rules finalized by European Commission despite industry calls for change
MedTech Europe pushed back against some of the requirements, but the Commission has retained disputed parts of the document.
By Nick Paul Taylor • July 8, 2022 -
Getinge recalls anesthesia machines due to cracked, broken suction power switches
The FDA labeled the recall a Class I event, marking Getinge’s fifth Class I recall since September.
By Ricky Zipp • July 7, 2022 -
Senators press FDA to finalize OTC hearing aids, accuse industry of 'astroturf' campaigns
The two cross-aisle politicians said they want to “expand access, reduce costs, and ensure a robust new market for safe and effective OTC hearing aids” and accused the device manufacturing lobby of “harming American consumers.”
By Elise Reuter • July 6, 2022 -
American Contract Systems' COVID-19 test recall gets Class I label from FDA
Off-site assembly by workers who may not have been properly trained prompted the company to recall COVID-19 tests amid concerns they may yield false results.
By Nick Paul Taylor • Updated July 6, 2022 -
AliveCor ECG patent ruling sets stage for block on Apple Watch imports
Apple Watch imports to the U.S. could be barred if ruling by International Trade Court judge is finalized; Judge says Apple Watch infringes two cardiogram patents.
By Nick Paul Taylor • July 5, 2022 -

 Retrieved from LiveMetric website on July 01, 2022
Retrieved from LiveMetric website on July 01, 2022
LiveMetric's blood-pressure 'smartwatch' device gets FDA clearance
The wristwatch-like device will be made available through health systems, insurers and self-insured employers to ease continuous monitoring of blood pressure and avoid white-coat syndrome.
By Nick Paul Taylor • July 1, 2022 -
 Retrieved from Siemsens Website on July 01, 2022
Retrieved from Siemsens Website on July 01, 2022
Intuitive secures FDA clearance for lung biopsy robot featuring Siemens' imaging tech
The FDA clearance is an “incremental positive” for Intuitive, wrote analysts at RBC Capital Markets, adding that they see Ion as “an important leg of growth for the company.”
By Nick Paul Taylor • July 1, 2022 -
As Philips works to replace or repair recalled devices, supply problems are slowing it down
Logistical challenges mean patients won’t know when they will get a replacement sleep apnea or ventilator device until “at best a few weeks before it is delivered,” Philips executives said.
By Ricky Zipp • June 30, 2022 -
Commercial labs to ramp up monkeypox testing in 'coming weeks'
The Food and Drug Administration said in a Wednesday meeting that public health labs currently have a throughput of 10,000 tests per week, and the addition of five reference labs will expand that capacity to 60,000 tests per week.
By Elise Reuter • June 29, 2022 -
Sleep apnea devices show 'very low' amount of degraded foam, Philips says
Philips found evidence of foam breaking down in about 2% of 60,847 returned first generation DreamStation devices. The company said the problem was more prevalent in devices that used an ozone cleaning method.
By Ricky Zipp • June 28, 2022 -
BD recalls emergency vascular access devices over risk of delayed care
Because patients who need intraosseous access are often critically ill, Becton Dickinson warned delays to care caused by the device problems could lead to death.
By Nick Paul Taylor • June 27, 2022 -
UnitedHealth's Optum looks to cut down on unnecessary lab testing
Optum says roughly 13 billion clinical lab tests are performed each year and 30% are unnecessary.
By Samantha Liss • June 27, 2022 -
Supreme Court overturns Roe v. Wade, ending constitutional right to abortion
26 states are certain or likely to end abortion rights, making abortion effectively illegal in half of the country, according to the Guttmacher Institute.
By Sydney Halleman • June 24, 2022 -
Baxter ventilator recall labeled Class I event by FDA after 2 deaths
Baxter initiated the recall after learning that an improper setup could reduce oxygen flow to in-home users, causing serious injury or death.
By Nick Paul Taylor • June 24, 2022 -
Patient death prompts another recall of Medtronic's HVAD System
The company initiated another recall for the troubled heart pump after a patient died when two batteries simultaneously stopped working, the company said, adding to a list of safety problems with the device.
By Ricky Zipp • June 23, 2022 -
FDA opens consultation on tissue removal devices to reduce cancer risk
The agency is seeking feedback on draft guidance to help manufacturers of tissue containment systems reduce risk that cancerous tissue could leak during procedures.
By Nick Paul Taylor • June 22, 2022 -
Deep Dive
As cross-state telemedicine waivers expire, virtual care advocates focus on long-term policy changes
There’s a complication: No one solution to the U.S.’ patchy physician licensing infrastructure has universal buy-in.
By Rebecca Pifer • June 22, 2022 -
Senseonics lands CE mark for 6-month CGM implant, teeing up Q3 launch in Europe
Senseonics is looking to the longer-lasting implant to re-energize its fight in a market dominated by Abbott Laboratories and Dexcom.
By Nick Paul Taylor • June 17, 2022 -
User fee package goes to Senate with lab-developed test, OTC hearing aid provisions
The Senate HELP committee passed its version of the FDA user fee bill by a 13-9 vote. It includes an overhaul of diagnostic testing regulations and a requirement to create a category of over-the-counter hearing aids.
By Elise Reuter • June 15, 2022 -
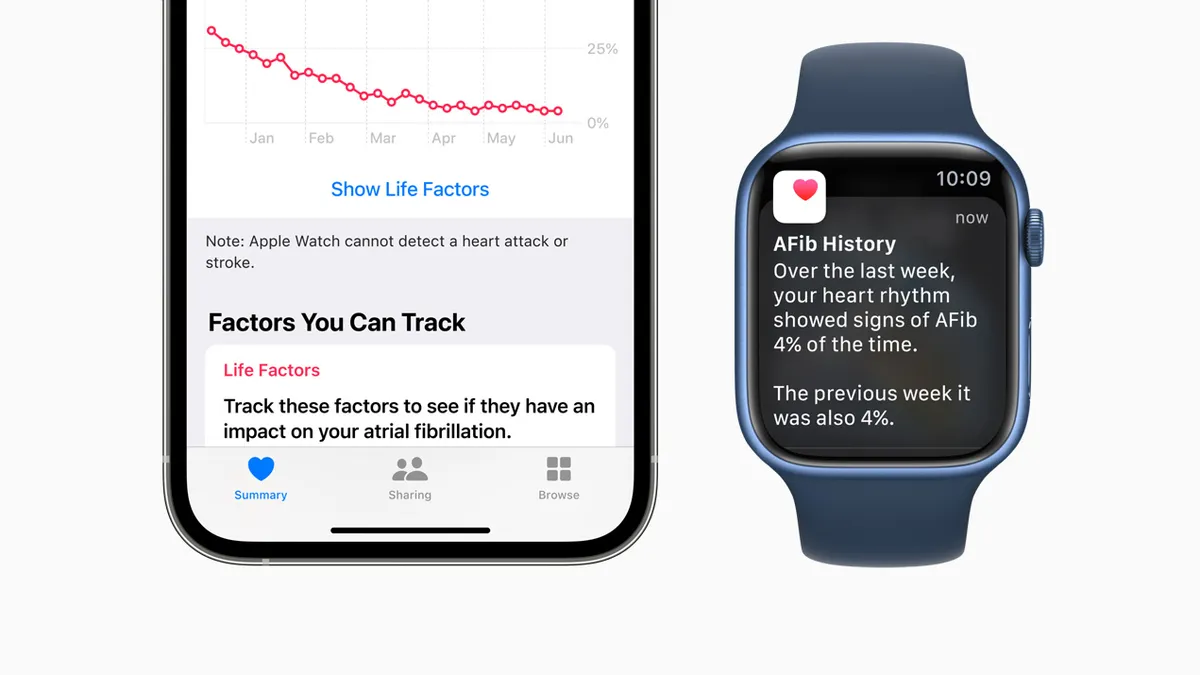
Apple Watch monitoring features for AFib, Parkinson's cleared by FDA
With the new feature, atrial fibrillation patients may have an easier way to track the frequency of the condition over time and see whether lifestyle changes may have positive effects.
By Nick Paul Taylor • June 14, 2022 -
Dräger's breathing system filter recall gets Class I label from FDA after ventilation obstruction
An obstruction in one of the company’s SafeStar 55 devices resulted in a patient being resuscitated after becoming hypoxic.
By Nick Paul Taylor • June 13, 2022