FDA: Page 25
-
Opinion
The importance of trust in AI as a medical device
Building trust with patients, as well as regulators, will be key to the adoption of AI in medical devices and diagnostics, argues Alison Dennis, a London-based partner with law firm Taylor Wessing.
By Alison Dennis • June 15, 2023 -
Huma hails 510(k) clearance as boost to digital health product development
The clearance permits Huma to host digital health tools from multiple creators that support screening, diagnosis, dosing recommendations and clinical decisions.
By Nick Paul Taylor • June 13, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
EPA’s proposed ethylene oxide regulations may cost sterilizers more than estimated: Moody’s
Large commercial sterilizers should be able to handle the increased costs, but smaller companies might have to sell, Moody’s analysts write.
By Elise Reuter • June 9, 2023 -
Cue Health wins first non-emergency authorization for COVID test, days after Goldman downgrade
The FDA’s marketing authorization for Cue’s COVID-19 molecular test could boost consumer access, but the company faces strong competition from more established diagnostic firms.
By Peter Green • June 7, 2023 -
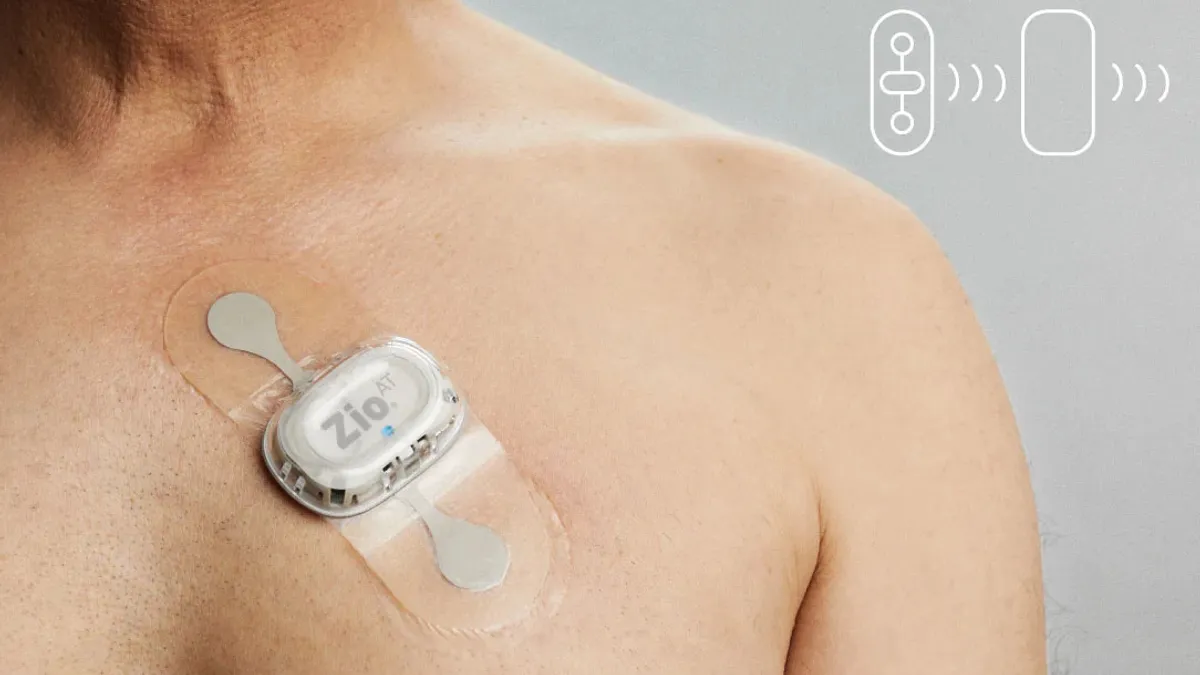
FDA: iRhythm targeted heart monitor at ‘high-risk’ patients without seeking clearance
William Blair analysts believe “customers are unlikely to change their usage of Zio AT [heart monitors] over time, making this a resolvable issue.”
By Nick Paul Taylor • June 7, 2023 -
Abiomed heart pump recall labeled Class I by FDA, no deaths reported
The recall, tied to specific sets of the Impella 5.5 with SmartAssist, comes just months after Johnson & Johnson paid $16.6 billion for Abiomed.
By Peter Green • Updated June 5, 2023 -
AdvaMed lobbies for medtech companies to be prioritized in global supply chain
The medtech trade group published a white paper outlining supply chain improvements needed to ensure availability of materials and parts for medical devices.
By Elise Reuter • June 2, 2023 -
FDA hits iRhythm with warning letter over problems at heart monitor facility
iRhythm can continue to manufacture and sell its products and does not expect the warning letter to materially affect its financial results.
By Nick Paul Taylor • June 1, 2023 -
Patient advocacy groups urge FDA to regulate lab-developed tests if VALID Act doesn’t pass
Legislation has been proposed in Congress to bring diagnostic tests under a single regulatory framework, but if it doesn’t pass, the FDA has authority to regulate lab-developed tests, the groups said.
By Elise Reuter • May 31, 2023 -
MedTech Europe sets out position on cybersecurity, making case for reliance on MDR
As the number of connected medical devices grows, the industry group argues that Europe’s new medtech regulations should remain “the primary avenue” for medical device cybersecurity.
By Nick Paul Taylor • Updated June 12, 2023 -
Illumina accused of ‘playing delay tactics’ to push back EU decision on divesting Grail: Financial Times
Delaying the decision to divest Grail could let Illumina sell the liquid biopsy business when the market is more favorable.
By Nick Paul Taylor • May 30, 2023 -
Q&A
Friday Q&A: Stanford Biodesign’s Makower on expediting Medicare coverage for breakthrough devices
The director and co-founder of the Stanford Byers Center for Biodesign has been urging CMS to get patients access to breakthrough devices.
By Susan Kelly • Updated May 26, 2023 -
Boston Scientific cancels $230M stent deal after regulators erect barriers to acquisition
The Federal Trade Commission said Boston Scientific took the action in response to investigations by its staff and overseas enforcement partners.
By Nick Paul Taylor • May 26, 2023 -
FDA teams with Veterans Health Administration to make medtech supply chains more resilient
Rather than amassing reserves of physical supplies, “digital stockpiles” would create a network of trusted suppliers that have the ability to make products at or near the point of use.
By Nick Paul Taylor • May 24, 2023 -
Beta Bionics wins FDA nod to challenge Medtronic for automated insulin pump market
Beta Bionics’ device, aimed at people with Type 1 diabetes, has eliminated most of the data entry required by competitors.
By Nick Paul Taylor • May 22, 2023 -

 Retrieved from Istock.
Retrieved from Istock.
Thermo Fisher gets FDA clearance for first blood test of severe preeclampsia risk
A study found the test is a better predictor of the risk of developing preeclampsia with severe features than standard clinical measures.
By Nick Paul Taylor • May 22, 2023 -

 Carol Highsmith. (2005). "The Apex Building" [Photo]. Retrieved from Wikimedia Commons.
Carol Highsmith. (2005). "The Apex Building" [Photo]. Retrieved from Wikimedia Commons.
FTC moves to strengthen Health Breach Notification Rule’s applicability to digital health apps
Regulators are increasingly leaning on the HBNR to crack down on the sharing of sensitive medical data, and the FTC is looking to strengthen its case.
By Rebecca Pifer Parduhn • May 18, 2023 -
Rapid AI adoption could cause medical errors, patient harm, WHO warns, urging oversight
Warning that caution is not being exercised with use of artificial intelligence in healthcare, the World Health Organization called for rigorous oversight of the new technology.
By Susan Kelly • May 17, 2023 -
Abbott wins FDA spinal cord stimulation approval to challenge Nevro for back pain market
The label expansion comes 16 months after Nevro won FDA approval in non-surgical refractory back pain and four months after Boston Scientific released data on its rival device.
By Nick Paul Taylor • Updated May 17, 2023 -
AdvaMed cites EPA’s own impact assessment to push back against ethylene oxide proposals
Medtech firms and environmental regulators are at loggerheads as the industry looks for a safe and cost-effective alternative to ethylene oxide for sterilizing reusable devices.
By Nick Paul Taylor • May 12, 2023 -
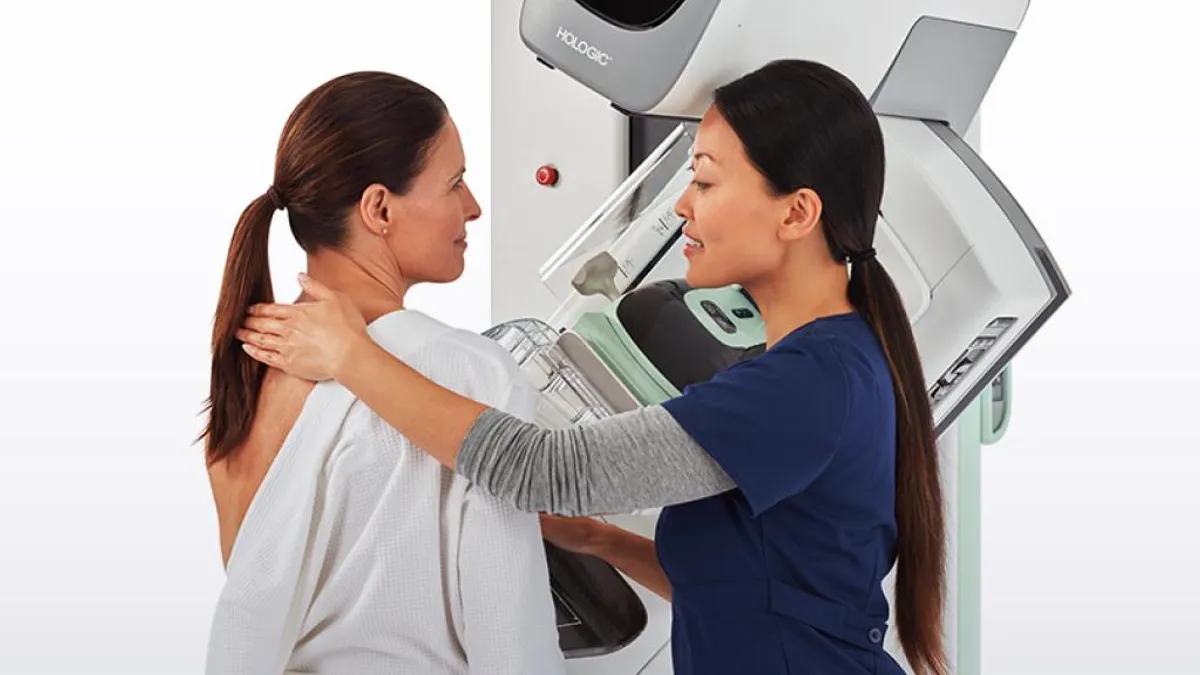
New breast cancer screening guidelines could boost mammography sales
Hologic, which makes mammography machines, could see an increase in its installed base, according to Needham analysts.
By Elise Reuter • May 9, 2023 -
FDA calls for ‘nimble’ regulation of ChatGPT-like models to avoid being ‘swept up quickly’ by tech
The head of the agency said the models are “ushering in the revolution that many of us were hoping for,” but cautioned that the sector will need to be regulated appropriately.
By Nick Paul Taylor • May 9, 2023 -
Boston Scientific gets DOJ subpoena for documents related to heart monitoring
The disclosure comes as cardiac monitoring device maker iRhythm also said it received a request from the Justice Department.
By Susan Kelly • May 5, 2023 -
CDC Director Walensky to step down in June
Rachel Walensky, who has served as director since 2001, was criticized for the federal government’s handing of the pandemic, announcing in August last year that she planned to reorganize the CDC.
By Sydney Halleman • May 5, 2023 -
FDA posts draft guidance on studying medical devices in decentralized clinical trials
Analysts at Cowen said the draft could reassure sponsors they can run decentralized trials without complicating approvals.
By Nick Paul Taylor • May 4, 2023