FDA: Page 24
-
GE’s nuclear imaging device can crush patients, FDA says, as Class I recall issued
GE HealthCare has told customers to stop using the machines until company technicians can inspect them and replace dangerous parts.
By Nick Paul Taylor • Feb. 17, 2023 -
AI devices need dedicated FDA regulatory pathway to reduce bias risk, say researchers
Scientists argue for a new regulatory process to prevent AI software from reinforcing existing health disparities.
By Nick Paul Taylor • Feb. 16, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Boston Scientific, MDMA call for more transparency and oversight of Medicare Advantage plans
Privately offered Medicare Advantage plans “do not often provide a clear reason for the denial of coverage, and rarely offer any visibility into the evidence and methodology,” trade group MDMA and Boston Scientific said.
By Nick Paul Taylor • Feb. 15, 2023 -
Opinion
Patent trolls’ destructive lawsuits are curbing US medtech innovation
The Patent and Trademark Office must help stop frivolous litigation that is harming the industry, argues former HHS secretary Tom Price.
By Tom Price • Feb. 10, 2023 -
82 more deaths linked to Philips’ recalled devices reported to FDA, bringing total to 346
The rate of death reports per month has risen consistently since Philips began the recall in the summer of 2021.
By Nick Paul Taylor • Feb. 10, 2023 -
Abbott balloon device partner Surmodics cuts jobs after FDA approval delay
The workforce reduction, one of a growing number in the medtech sector, comes after the agency requested more data in a premarket review of the company’s device to treat narrowed leg arteries.
By Susan Kelly • Feb. 9, 2023 -
Biden zeroes in on drug costs during State of the Union address
The president called for extending Medicare's $35 insulin price cap to all Americans and blasted “big pharma” for record profits while at the same time “unfairly charging people hundreds of dollars.”
By Shannon Muchmore • Feb. 8, 2023 -
Trade group pushes back on FDA’s clinical decision support guidance
The Clinical Decision Support Coalition is asking the agency to rescind its guidance and propose new rules that change how software tools are regulated as medical devices.
By Elise Reuter • Feb. 8, 2023 -
UK health system, under strain, lays out medtech strategy
The state-run National Health Service, now facing a strike by nurses and paramedics, hopes new technologies will help solve some of its long-term challenges to improve patient care.
By Susan Kelly • Feb. 7, 2023 -
FTC orders GoodRx to stop sharing users’ health data with advertisers, issues $1.5M fine
Regulators said they are putting the industry on watch with the enforcement against GoodRx, which allegedly collected data, including patient medications, and shared it without user consent.
By Rebecca Pifer • Updated Feb. 1, 2023 -
FDA device center’s 2022 report reveals 37% drop in annual breakthrough designations
The annual report also highlighted the CDRH’s work on pulse oximeters, Philips’ recall of sleep and respiratory devices, cybersecurity and artificial intelligence.
By Nick Paul Taylor • Jan. 31, 2023 -
Philips will cut 6,000 more jobs to reduce costs after company swung to net loss in Q4
New CEO Roy Jakobs said Philips has been spreading its resources too thinly and “can't and won't be selling everything everywhere anymore.”
By Nick Paul Taylor • Jan. 30, 2023 -
Medtech trends in 2023
Medtech regulation outlook in 2023: Faster approvals among priorities as AI moves to fore
Leaders in the medtech field weigh in on the prospects and challenges for product regulation in 2023.
By Peter Green • Updated Jan. 26, 2023 -
Getinge heart pump gets third Class I recall after patient death
Customers were alerted to problems with Cardiosave devices late last year after Getinge received complaints about an issue linked to four serious injuries and one death.
By Nick Paul Taylor • Jan. 26, 2023 -
Insulet reports personal-data leak of 29,000 insulin pump customers
In a recall communication to users, Insulet shared information with its website performance and marketing partners through cookies and other trackers embedded in its website.
By Nick Paul Taylor • Jan. 24, 2023 -
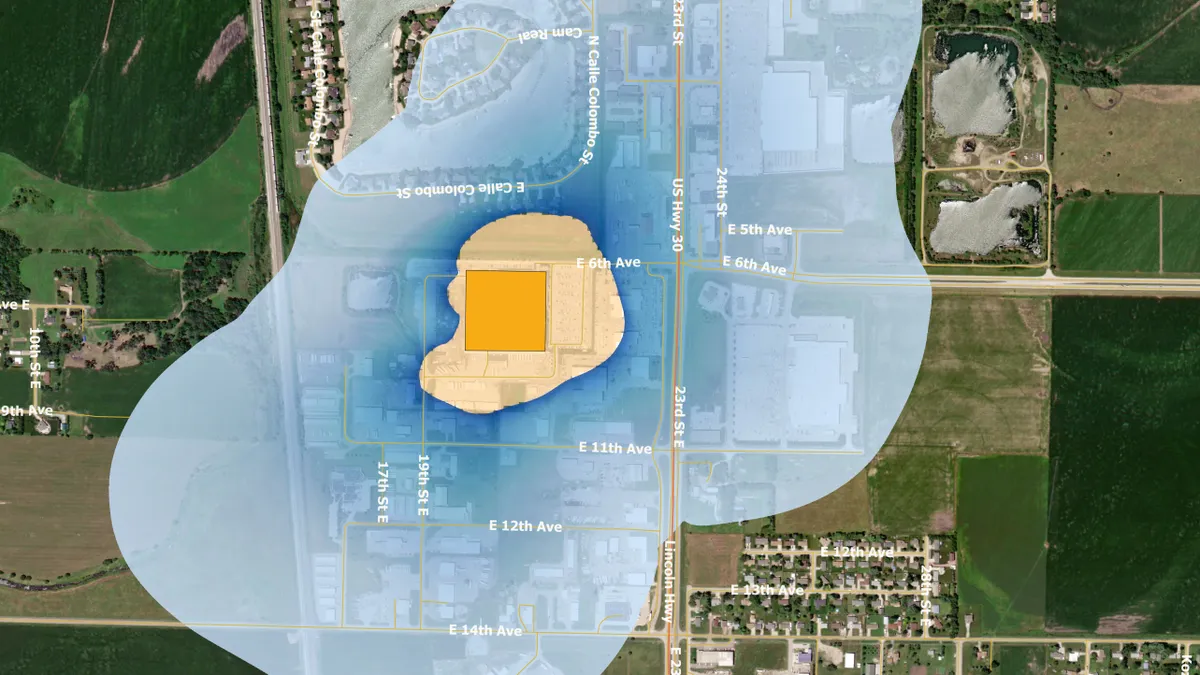
 EPA. (2022). "https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/forms/columbus-nebraska-becton-dickinson-pharmaceutical" [Photo]. Retrieved from EPA.gov.
EPA. (2022). "https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/forms/columbus-nebraska-becton-dickinson-pharmaceutical" [Photo]. Retrieved from EPA.gov.
Ethylene oxide regulation may disrupt device supply, AdvaMed warns White House
The trade group has asked President Joe Biden to consider the potential threat to patient care if facilities are shut down.
By Nick Paul Taylor • Jan. 23, 2023 -
FDA demands additional data on Abbott rival to Medtronic balloon, setting back approval timeline
Abbott’s partner Surmodics is “evaluating options” to reduce its spending while preparing a response to the regulator’s requests.
By Nick Paul Taylor • Jan. 20, 2023 -
Medtech hazards: Problems with Philips’ recall put safety of home-use devices at top of danger list
Patient safety group ECRI is challenging manufacturers to provide easy-to-follow device registration instructions and write simply worded recall notices.
By Nick Paul Taylor • Jan. 19, 2023 -
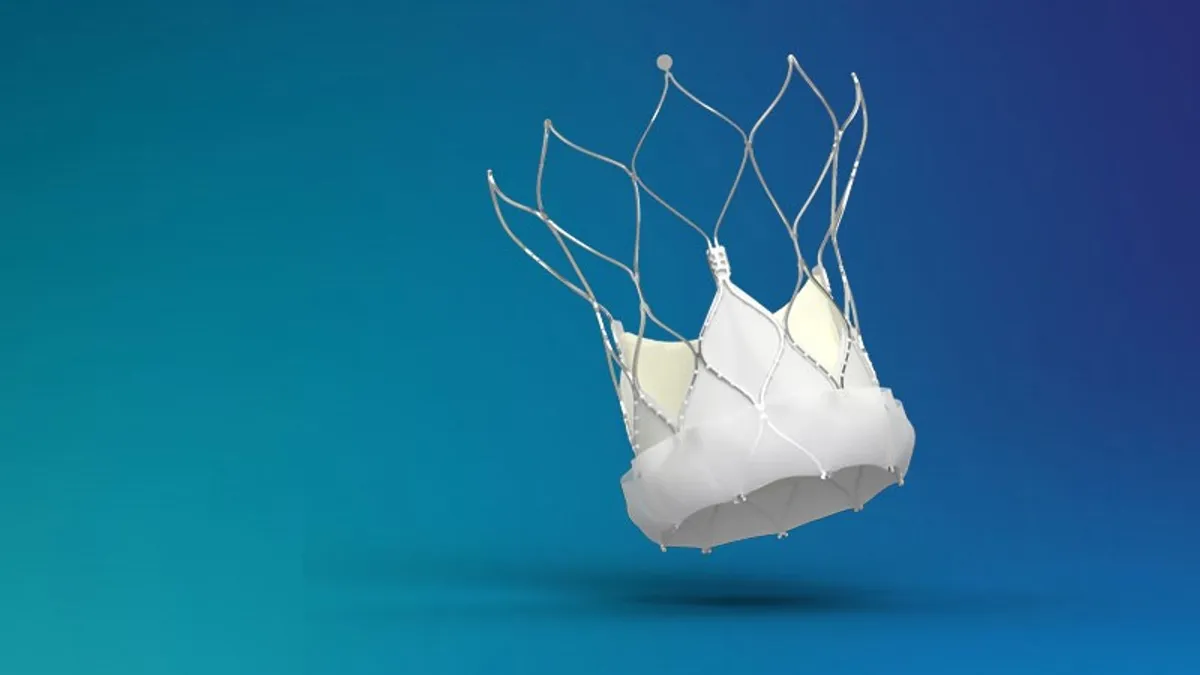
Abbott’s FDA approval for TAVI device tees up challenge to Edwards, Medtronic: analysts
Abbott has captured about 9% of the European market and aims to become a “credible third player” in the U.S.
By Nick Paul Taylor • Jan. 18, 2023 -
Rethinking 510(k): Studies show risk of using recalled devices as predicates for FDA clearance
Two studies found devices cleared through the FDA’s 510(k) pathway that listed recalled predicate devices were more likely to face recalls themselves.
By Nick Paul Taylor • Jan. 11, 2023 -
European Commission formalizes plan to extend MDR transition out to 2027, 2028
Having accepted that notified body capacity “remains insufficient,” the Commission wants to give manufacturers more time to get their devices designated under MDR.
By Nick Paul Taylor • Jan. 9, 2023 -
Vivos bounces back from FDA rejection to land 510(k) clearance for sleep apnea treatment
Shares in Vivos increased by more than 150% on the day of the news.
By Nick Paul Taylor • Jan. 6, 2023 -
Roche receives first European IVDR certificate for companion diagnostic
Receipt of the certificate clears Roche to sell a companion diagnostic for immune checkpoint inhibitors in the European Union.
By Nick Paul Taylor • Jan. 5, 2023 -
Diagnostic testing reform missing from Congress’ year-end spending bill
A provision that would have brought in-vitro diagnostic tests and lab-developed tests under one regulatory framework was not included in the omnibus spending bill.
By Elise Reuter • Dec. 21, 2022 -
MDR costs are driving manufacturers to pull products from European market: report
The report adds to evidence that makers of products used for pediatric patients and rare diseases have been particularly hard hit.
By Nick Paul Taylor • Dec. 20, 2022