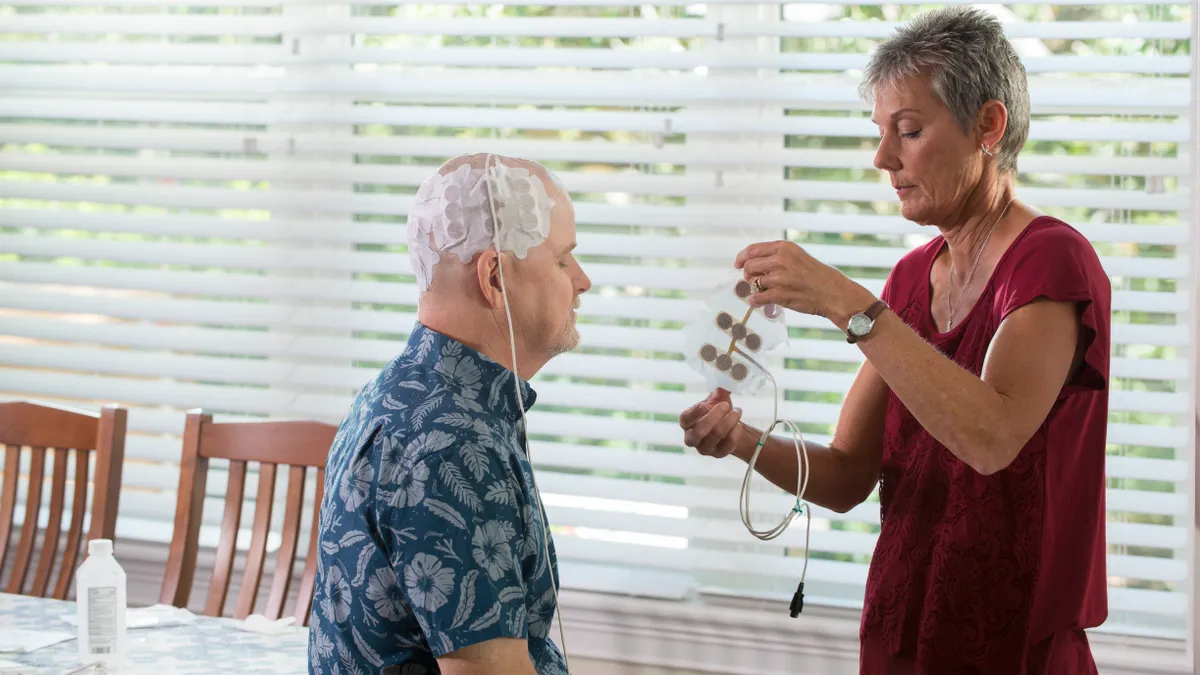Medical Devices: Page 140
-
FDA issues device sterilization warning letter
The notice accuses Innovative Sterilization Technologies of promoting the sterilization product in applications outside of the scope of its 510(k) clearance.
By Nick Paul Taylor • Aug. 21, 2019 -
FDA approves MRI labeling for Boston Scientific DBS system
CEO Michael Mahoney touted the growth of the company's deep brain stimulation products to investors during its second quarter earnings call.
By David Lim • Aug. 20, 2019 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Conformis sues Zimmer Biomet over patent infringement
Knee implant maker Conformis is seeking triple damages amid its claim that Zimmer Biomet infringed multiple of its patents for patient-specific instrument systems and techniques for joint replacement surgery.
By Susan Kelly • Aug. 20, 2019 -
Medtronic to ramp up TAVR, robotics programs after Street-beating Q1
Building on momentum from the Mazor Robotics integration, the medtech giant teased a Sept. 24 investor day that will feature a demonstration of its forthcoming soft tissue robotic surgery platform.
By Maria Rachal • Aug. 20, 2019 -
Italy's IMQ fourth notified body designated in EU
The firm joins Germany's Dekra and TÜV SÜD, as well as the United Kingdom's BSI, as the only four bodies designated less than a year before the new regulations take effect in May 2020.
By David Lim • Aug. 20, 2019 -
Fusion-less scoliosis device from Zimmer Biomet earns HDE
Using a flexible tether implant that applies tension to correct the curvature, the device is an alternative to fusion surgery in children and adolescents, designed to preserve range of motion in the spine.
By Susan Kelly • Aug. 19, 2019 -
FDA approves neuromodulation device for heart failure
The system was one of the first devices to win the agency's breakthrough status, which helped accelerate development and approval, according to manufacturer CVRx.
By Susan Kelly • Updated Aug. 19, 2019 -
Boston Scientific closes acquisition of BTG
The medtech giant is betting that having the subsidiary will make it the "category leader" in interventional oncology tools and products.
By Maria Rachal • Updated Aug. 19, 2019 -
Edwards, Medtronic win expanded indications for low-risk TAVR patients
Roughly 165,000 low-risk patients per year in the U.S., Western Europe and Japan could now be eligible for the procedure, Medtronic estimates, a population both medtechs will be keen to treat.
By David Lim , Maria Rachal • Updated Aug. 16, 2019 -
Tidepool CEO talks Pre-Cert era, interoperability
Amazon and Pixar software veteran Howard Look spoke to MedTech Dive about working through FDA's software development evaluation pilot and the implications of an insulin dosing app that works with many brands' devices.
By Maria Rachal • Updated Aug. 16, 2019 -
V-Wave gets 2nd breakthrough designation for interatrial shunt
Building on its heart failure indication, the J&J- and Edwards-backed medtech is conducting an early feasibility study of the device in patients with pulmonary arterial hypertension.
By Susan Kelly • Updated Sept. 12, 2019 -
Mercy expands RWE program to capture data from other providers
The expansion of the network follows real-world evidence deals with BD, Johnson & Johnson and Medtronic.
By Nick Paul Taylor • Aug. 16, 2019 -
UK plans for express medical products shipping service ahead of Brexit
The announcement of the shipping service comes as the government, led by new prime minister Boris Johnson, steps up no-deal scenario preparations and downplays risks.
By Nick Paul Taylor • Aug. 16, 2019 -
Hearing industry calls Bose self-fit hearing aid study flawed in complaint to FDA
The Hearing Industries Association letter argues the device's Phase II clinical study "does not provide enough evidence of effectiveness of the self-fitting method given the study's initial reliance on professionals for fitting."
By David Lim • Aug. 15, 2019 -
FDA taps Type A aortic dissection stent for breakthrough program
Ascyrus Medical's leg up from U.S. regulators follows Health Canada's approval of its device for a rare but potentially fatal condition last December and CE marking in February.
By Maria Rachal • Aug. 15, 2019 -
Abbott poised to profit from CMS review of TMVR coverage
Analysts at Cowen estimate CMS coverage in a newly considered indication could triple the number of patients eligible for transcatheter mitral valve repair.
By Nick Paul Taylor • Aug. 15, 2019 -
Industry backs Australia's device identifier plan with caveats
The responses showed broad support for a unique device identification system based on global guidelines, but the Therapeutic Goods Administration also faced some pushback from J&J and Roche.
By Nick Paul Taylor • Aug. 15, 2019 -
FDA hits medicine cabinet and cart maker Talon with warning letter
The agency found 11 violations of quality system regulations, ranging from failure to follow audit principles and perform risk analysis to inadequate procedures covering design changes and control of nonconforming products.
By Susan Kelly • Aug. 14, 2019 -
Analysts predict big jump in continuous glucose monitor use
Abbott and Dexcom are likely to dominate the CGM market in the long term. Medtronic is expected to remain the leader in insulin pumps.
By Susan Kelly • Aug. 14, 2019 -
Stryker acquires maker of device sterilization systems
The deal comes against a backdrop of heightened regulatory scrutiny of duodenoscope reprocessing after a spike in contamination cases and the closure of a Sterigenics plant in Illinois that sterilized a wide range of devices.
By Susan Kelly • Aug. 13, 2019 -
Australia weighs in on paclitaxel-coated balloons
The Therapeutic Goods Administration said data on adverse events in Australia haven't shown a signal like the one described by FDA.
By Susan Kelly • Aug. 13, 2019 -
Concept Medical nabs breakthrough designation for sirolimus-coated balloon for PAD
If granted FDA marketing authorization, the device may compete with paclitaxel devices sold by BD, Medtronic, Boston Scientific, Philips and Cook Medical.
By David Lim • Aug. 13, 2019 -
Two more notified bodies designated in EU, but pace still lags
The public database NANDO lists German company Dekra Certification as one of the two notified bodies.
By Dana Elfin • Updated Aug. 14, 2019 -
China grants innovative device designation to Novocure's Optune
Novocure's Chinese partner now gets the opportunity for more contact with regulators and an expedited review in a market estimated at $96.3 billion this year, up from $53.6 billion in 2016.
By Nick Paul Taylor • Aug. 13, 2019 -
Ambu to pay $3.3M to settle False Claims Act allegations
The Danish medical device maker is accused of manufacturing products in China and Malaysia and knowingly selling them to U.S. government agencies in violation of the 1979 Trade Agreements Act.
By Susan Kelly • Aug. 12, 2019