Medical Devices: Page 139
-
Summer slowdown drags on for De Novos
The FDA has not announced a De Novo clearance in 12 weeks. Two-thirds of the way through 2019, pacing suggests the overall number of novel device approvals won't match 2018's high.
By Maria Rachal • Aug. 30, 2019 -
McKesson, Philips devices flagged by DHS for cyber vulnerabilities
Certain cardiovascular IT systems and ultrasound devices could be exploited by hackers, the Department of Homeland Security wrote in separate notices.
By Nick Paul Taylor • Aug. 30, 2019 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
AdvaMed wins some, loses some in FDA guidance on managing uncertainty
While the agency tweaked parts of the document in line with feedback from AdvaMed and other groups, it maintained its plan to hold advisory committee meetings on certain postmarket data and kept other contested proposals.
By Nick Paul Taylor • Aug. 30, 2019 -
Medline, Vantage ethylene oxide emissions caused cancer, lawsuits claim
Commercial sterilizer Sterigenics, which was forced to shut down a Willowbrook medical device sterilizing plant in February, is defending itself against at least 32 lawsuits in the same state court over similar claims.
By David Lim • Aug. 29, 2019 -
Surmodics passes milestone in trial of Abbott-licensed paclitaxel-coated balloon
Surmodics completed enrollment sooner than some analysts expected on a trial comparing its paclitaxel-coated balloon to Medtronic's IN.PACT Admiral.
By Nick Paul Taylor • Aug. 29, 2019 -
Elekta buys ProKnow to boost digital radiotherapy strategy
The Swedish company says ProKnow's capabilities are of particular value to its large customers, potentially helping give it an edge over rivals such as Varian Medical Systems.
By Nick Paul Taylor • Aug. 29, 2019 -
Life Spine talks up global expansion weeks after DOJ hit
The micro invasive spine tech maker says its products are now sold in 30 countries, including recent additions in South America.
By Nick Paul Taylor • Aug. 29, 2019 -
Medtronic's Ishrak set to retire after nearly decade as CEO
The leadership shuffle paves the way for Restorative Therapies Group head Geoff Martha, who led Medtronic's nearly $43 billion takeover of Covidien in 2014, to take the helm in time for the medtech giant's next fiscal year.
By Maria Rachal • Aug. 28, 2019 -
AtriCure gets expanded FDA label for atrial exclusion device
AtriCure said it has strong growth from the AtriClip FLEX.V device and is confident growth rates for appendage management products will continue.
By Nick Paul Taylor • Aug. 28, 2019 -
FDA approves pediatric spine device via humanitarian pathway
The device is a less invasive alternative to spinal fusion for adolescents with idiopathic scoliosis.
By Nick Paul Taylor • Aug. 28, 2019 -
Titan Medical delays 510(k) submission until 2020 amid cash burn concerns
The company plans to use the delay to spread out its spending while working to implement the robotic surgery system's sterile instrument interface components, software improvements and training tools.
By David Lim • Aug. 27, 2019 -
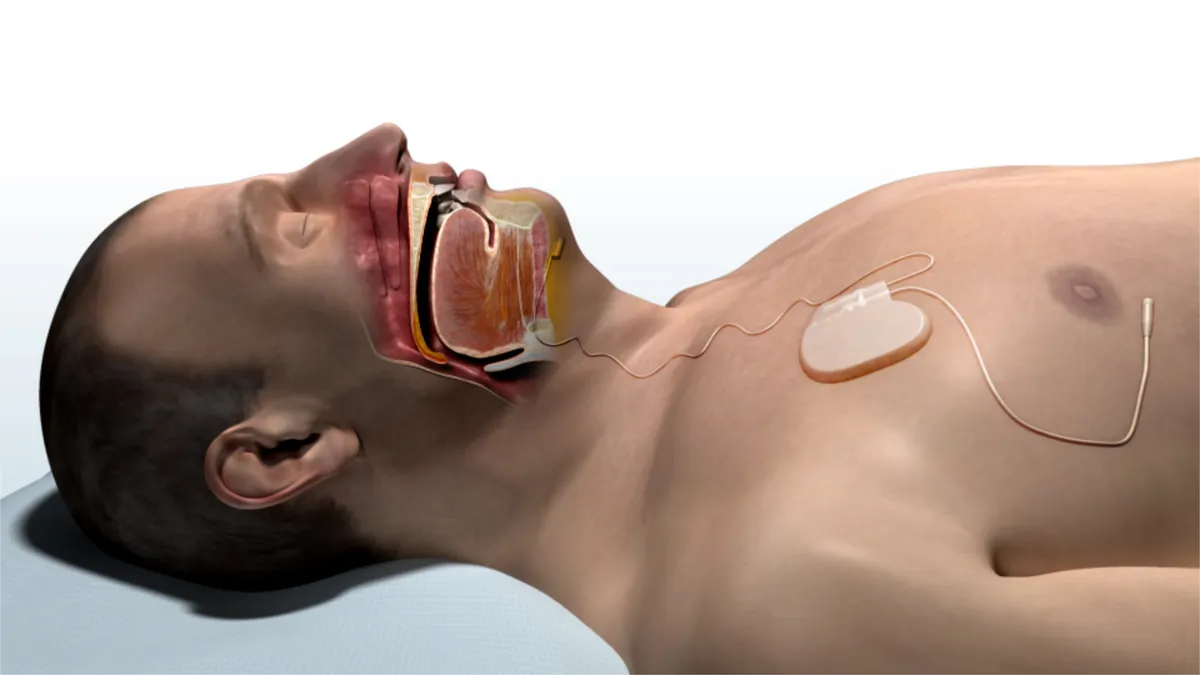
Medicare contractors back use of Inspire's sleep apnea device
Inspire Medical has now received draft decisions from four different Medicare contractors for the sleep apnea therapy, which could eliminate reimbursement "fatigue factors" the company says are limiting use of the product.
By Nick Paul Taylor • Updated Sept. 3, 2019 -
Breast implant makers vie for customers amid Allergan recall
Even with greater awareness of cancer and chronic illness linked to breast implants, consumer interest does not appear to have waned.
By Maria Rachal • Aug. 26, 2019 -
Dexcom beats WaveForm's patent suit over CGM sensors
A U.S. District Court judge's decision ends the ongoing dispute between San Diego-based continuous glucose monitor maker Dexcom and WaveForm Technologies, a subsidiary of blood glucose meter manufacturer AgaMatrix.
By Dana Elfin • Aug. 26, 2019 -
Big data, cybersecurity among top FDA device center priorities
Reprocessing of devices and biocompatibility are also addressed in a report intended to incorporate new tech into regulatory decisions.
By David Lim • Aug. 23, 2019 -
Heart attack device earns FDA's breakthrough nod
Miracor Medical began a 144-patient clinical trial in July to examine effectiveness of its device. Earlier studies ran into hurdles.
By Nick Paul Taylor • Aug. 23, 2019 -
Medicare draft decisions may boost Natera, Myriad tests
Cowen analysts called proposed local coverage for Natera's colorectal cancer test as "materially broader and quicker than expected," while documents on pharmacogenomic tests gave a mixed outlook for Myriad Genetics' products.
By Nick Paul Taylor • Aug. 23, 2019 -
FDA flags recall of Edwards TAVR delivery system tied to 1 death, 17 injuries
Analysts characterized FDA's Class I notice Thursday of Edwards' recall, based on an issue highlighted in an urgent field safety notice last month, as a noisy headline not likely to meaningfully affect revenue.
By Maria Rachal • Updated Aug. 23, 2019 -
Industry sounds alarm on EU single-use device reprocessing regs
There remains a risk that the European Commission will miss its November target and fail to finalize the draft policy before MDR comes into force.
By Nick Paul Taylor • Updated Aug. 27, 2019 -
AdvaMed warns White House sterilization regs pose device shortage risk
The trade association's chief lobbyist told administration officials in a meeting an effective ban on ethylene oxide as a sterilant "poses an imminent public health threat through shortages of medical devices."
By David Lim • Aug. 21, 2019 -
ViewRay touts promising early results from localized prostate cancer trial after disappointing quarter
Weeks after cutting revenue targets for 2019, the company reported low incidence of early gastrointestinal and genitourinary toxicity in patients who received the magnetic resonance-guided radiation therapy.
By Maria Rachal • Aug. 21, 2019 -
FDA issues device sterilization warning letter
The notice accuses Innovative Sterilization Technologies of promoting the sterilization product in applications outside of the scope of its 510(k) clearance.
By Nick Paul Taylor • Aug. 21, 2019 -
FDA approves MRI labeling for Boston Scientific DBS system
CEO Michael Mahoney touted the growth of the company's deep brain stimulation products to investors during its second quarter earnings call.
By David Lim • Aug. 20, 2019 -
Conformis sues Zimmer Biomet over patent infringement
Knee implant maker Conformis is seeking triple damages amid its claim that Zimmer Biomet infringed multiple of its patents for patient-specific instrument systems and techniques for joint replacement surgery.
By Susan Kelly • Aug. 20, 2019 -
Medtronic to ramp up TAVR, robotics programs after Street-beating Q1
Building on momentum from the Mazor Robotics integration, the medtech giant teased a Sept. 24 investor day that will feature a demonstration of its forthcoming soft tissue robotic surgery platform.
By Maria Rachal • Aug. 20, 2019