Medical Devices: Page 14
-
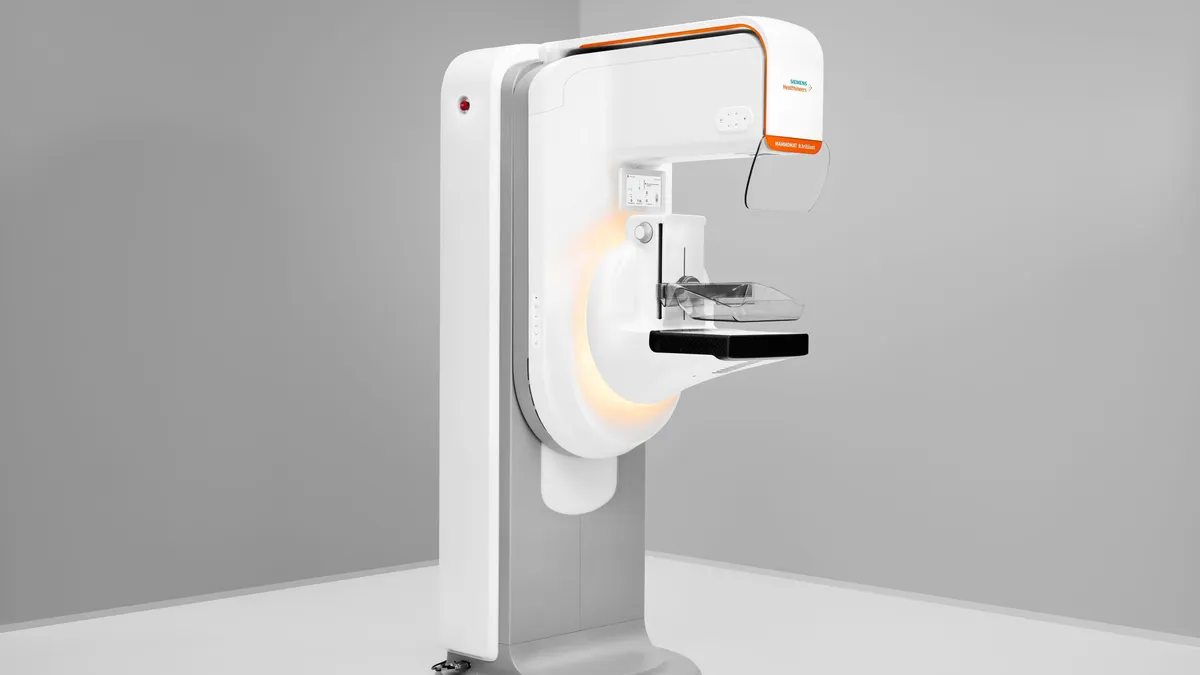
Siemens Healthineers wins FDA approval for 3D mammography system
The 3D capabilities are part of Siemens’ first completely redesigned mammography platform in over a decade.
By Nick Paul Taylor • Oct. 2, 2024 -
GE Healthcare gets FDA nod for new PET imaging agent
Called Flyrcado, the radiotracer could help improve cardiac imaging accuracy in patients with a high body mass index and women, the company said.
By Susan Kelly • Oct. 1, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Top medtech conferences in 2025
The lineup includes industrywide conferences and events covering the latest medtech trends in diabetes, orthopedics, cardiac care and surgical robotics.
By Ricky Zipp • Updated Jan. 23, 2025 -
Synchron’s brain-computer interface tech meets safety goal
The six-patient trial also indicated the device worked, according to Synchron, with the conversion of brain signals to motor outputs allowing people to perform digital tasks.
By Nick Paul Taylor • Oct. 1, 2024 -
Baxter closes dialysis solutions facility due to Hurricane Helene damage
More than 2,500 people work at the facility, which is Baxter’s largest manufacturing site. The company is still working to fully assess the damage.
By Elise Reuter • Updated Sept. 30, 2024 -
Establishment Labs wins FDA approval for Motiva breast implants
Motiva is the first new breast implant to receive premarket approval since 2013, according to Establishment Labs, and enters a market reshaped by safety issues linked to other products.
By Nick Paul Taylor • Sept. 30, 2024 -
What’s next at Masimo after CEO Kiani’s exit?
Investors are waiting to learn the next steps for the patient monitoring company after a hard-fought proxy battle, including how a separation of its consumer business could play out.
By Susan Kelly • Sept. 30, 2024 -
Remote patient monitoring in Medicare needs more oversight: OIG
Regulators say Medicare needs more data and oversight to avoid fraud and misuse. Digital health advocates argue the service is still crucial for managing chronic conditions.
By Emily Olsen • Sept. 27, 2024 -
Mendaera raises $73M to develop handheld robotics platform
Fred Moll, founder of surgical robotics firms Intuitive Surgical and Auris Health, participated in in the financing round, along with previous Auris investors.
By Nick Paul Taylor • Sept. 27, 2024 -
Lawmakers call for investigation of former FDA device director
The letter to the health department watchdog follows a New York Times report that raised concerns about Jeff Shuren’s potential conflicts of interest as head of the FDA’s device center.
By Elise Reuter • Sept. 26, 2024 -
FDA proposes reclassifying hepatitis B assays
The FDA plans to move the assays from higher-risk class III devices to class II, making them eligible for the 510(k) pathway.
By Nick Paul Taylor • Sept. 26, 2024 -
Medtronic, Siemens Healthineers partner on spine surgery
Siemens Healthineers will integrate its robotic X-ray imaging system with Medtronic’s spine surgery suite.
By Elise Reuter • Sept. 25, 2024 -
Masimo CEO, founder Kiani resigns
Michelle Brennan was named acting CEO of the patient monitoring company following Joe Kiani’s defeat in a proxy battle with an activist investor.
By Susan Kelly • Sept. 25, 2024 -
FDA names new head of medical device evaluation and quality
Ross Segan was chosen to lead the office, which handles premarket authorizations and recalls, in the agency’s Center for Devices and Radiological Health.
By Susan Kelly • Sept. 24, 2024 -
Recalled heart devices had limited clinical testing, study finds
Just 30 of 157 heart devices with Class I recalls underwent premarket clinical testing, according to a study published in the Annals of Internal Medicine.
By Elise Reuter • Sept. 24, 2024 -
Route 92 Medical raises $50M to fund global sales of stroke devices
The latest investment extends an earlier funding round, bringing the total raised to $82 million. Novo Holdings, the controlling shareholder of Novo Nordisk, joined returning investors.
By Nick Paul Taylor • Sept. 24, 2024 -
Stryker adds devices to remove brain tumors, clots with Nico buy
Nico sells devices for navigating through the brain, visualizing the organ, removing tumors and clots and collecting tissue.
By Nick Paul Taylor • Sept. 23, 2024 -
FDA posts draft guidance on biocompatibility testing of devices
To promote consistency and reliability in premarket submissions, the FDA shared approaches for device biocompatibility analysis.
By Nick Paul Taylor • Sept. 23, 2024 -
Deep Dive
4 steps to minimize the threat of legacy medical devices
Older medical devices with unsupported software pose cybersecurity threats that regulators and industry are struggling to solve. Here are four steps experts say can help mitigate risks.
By Ricky Zipp • Sept. 23, 2024 -
Abbott ramps up Libre 3 production amid shortages
Some people are facing delays in filling prescriptions for the glucose sensor. Abbott expects to resolve the problem soon.
By Elise Reuter • Sept. 20, 2024 -
Masimo investor claims proxy war win as CEO Kiani fails to retain board seat
Politan Capital Management said its candidates captured two board seats in Thursday’s shareholder vote, citing a preliminary analysis.
By Susan Kelly • Sept. 20, 2024 -
Jury sides with Axonics in Medtronic patent dispute
Meanwhile, Boston Scientific is still waiting for antitrust regulators to conclude their review of its proposal to buy Axonics, which was announced in January.
By Elise Reuter • Sept. 19, 2024 -
Masimo proxy fight nears end as shareholder vote looms
Chairman and CEO Joe Kiani’s board seat is on the line at today's shareholder meeting.
By Susan Kelly • Sept. 19, 2024 -
Zimmer pulls hip implant off the market due to fracture risk
The company plans to phase out use of its CPT Hip System by December, but the FDA is still concerned about implants in new patients.
By Elise Reuter • Sept. 18, 2024 -
Merit Medical inks $210M takeover of Cook’s lead management business
Merit predicts the portfolio, which includes devices used in procedures to remove or replace heart rhythm device leads, will add $40 million a year to its sales.
By Nick Paul Taylor • Sept. 18, 2024