Medical Devices: Page 13
-
Solventum names Egeland as chief medical officer
Ryan Egeland, former CEO and co-founder of Crossfire Medical, rounds out the leadership team for the 3M spinoff.
By Elise Reuter • Oct. 11, 2024 -
Abbott enrolls pivotal PFA study 4 months ahead of schedule
The company reported the enrollment milestone alongside news that it received clearance to sell its Advisor HD Grid X Mapping Catheter and started a trial of its Tactiflex Duo Ablation Catheter.
By Nick Paul Taylor • Oct. 11, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
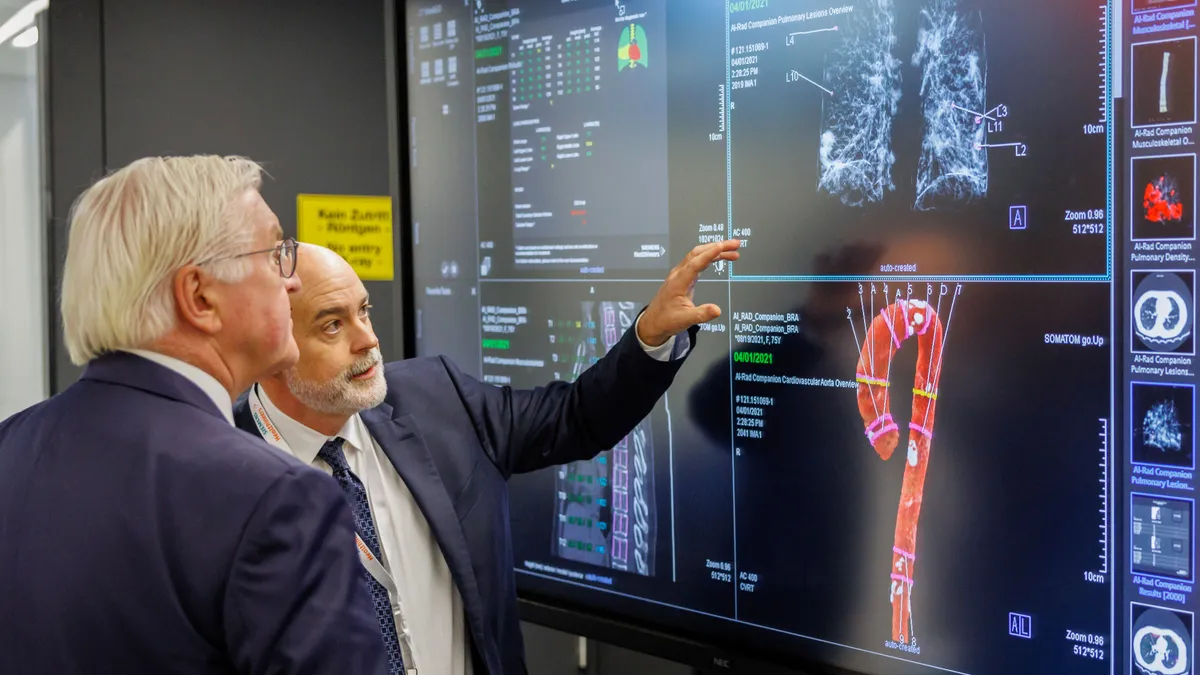
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Shiratronics raises $66M for trial of neuromodulation migraine device
The funding will support the trial, premarket approval application and initial commercial launch of a system that could interrupt or change the transmission of pain signals to the brain.
By Nick Paul Taylor • Oct. 10, 2024 -

 Retrieved from Aerial Lens on October 10, 2024
Retrieved from Aerial Lens on October 10, 2024
Baxter ups IV fluid allocations amid supply shortages
As hospitals around the U.S. report IV fluid shortages, Baxter has increased supply allocations for direct customers and distributors. It plans on returning to 90% to 100% allocation of certain supplies by the end of 2024.
By Ricky Zipp • Oct. 10, 2024 -
CMR Surgical CEO Bose steps down
Massimiliano Colella, who joined the surgical robotics firm in late 2023, was named interim CEO.
By Susan Kelly • Oct. 10, 2024 -
Deep Dive
The number of AI medical devices has spiked in the past decade
The FDA has authorized nearly 1,000 medical devices with artificial intelligence features as companies including GE Healthcare and Siemens Healthineers build out their AI efforts.
By Elise Reuter , Jasmine Ye Han • Oct. 9, 2024 -
AHA asks Biden administration for relief amid IV fluid shortage from Hurricane Helene
Hospitals have been contending with shortages of the life-saving supplies after Hurricane Helene damaged Baxter's manufacturing plant in North Carolina, according to the American Hospital Association.
By Susanna Vogel • Oct. 9, 2024 -

 Retrieved from Aerial Lens on October 08, 2024
Retrieved from Aerial Lens on October 08, 2024
Baxter says no structural damage at North Carolina site hit by Hurricane Helene
The facility also has access to water and power. Baxter expects to share production plans over the next two weeks.
By Elise Reuter • Oct. 8, 2024 -
CMS begins national coverage review for tricuspid repair
The agency will consider national reimbursement for new tricuspid transcatheter repair devices, including Abbott’s Triclip, in Medicare patients with a leaky tricuspid heart valve.
By Susan Kelly • Oct. 8, 2024 -
Mercury Medical recalls emergency resuscitators
Healthcare professionals use the devices to provide newborns and infants with emergency breathing support.
By Nick Paul Taylor • Oct. 8, 2024 -
Smiths recalls ventilators over risk of oxygen flow disruption
Smiths has grappled with multiple quality issues and recalls over the past few years. Two recent recalls focus on problems with portable ventilators.
By Elise Reuter • Oct. 7, 2024 -
Medtronic recalls Minimed insulin pumps for reduced battery life
The company said it received 170 reports of hyperglycemia and 11 reports of diabetic ketoacidosis in the U.S. that could be linked to the problem.
By Susan Kelly • Oct. 7, 2024 -

 Retrieved from Aerial Lens on October 04, 2024
Retrieved from Aerial Lens on October 04, 2024
Baxter unsure when North Carolina facility hit by Hurricane Helene will be operational
As access challenges to the site continue, Baxter has limited purchases of certain products to avoid stockpiling, manage inventory and minimize disruption to patient care.
By Ricky Zipp • Oct. 4, 2024 -
Independent lab fires back after Philips sues over testing results
PSN Labs called for a jury trial, claiming Philips is attempting to deflect attention away from its own “failures, negligence, concealment and recklessness.”
By Elise Reuter • Oct. 3, 2024 -
BD agrees to settle most of its hernia mesh litigation
The settlement terms are confidential, BD said. The company disputes the lawsuit’s allegations and backs its product designs.
By Susan Kelly • Oct. 3, 2024 -
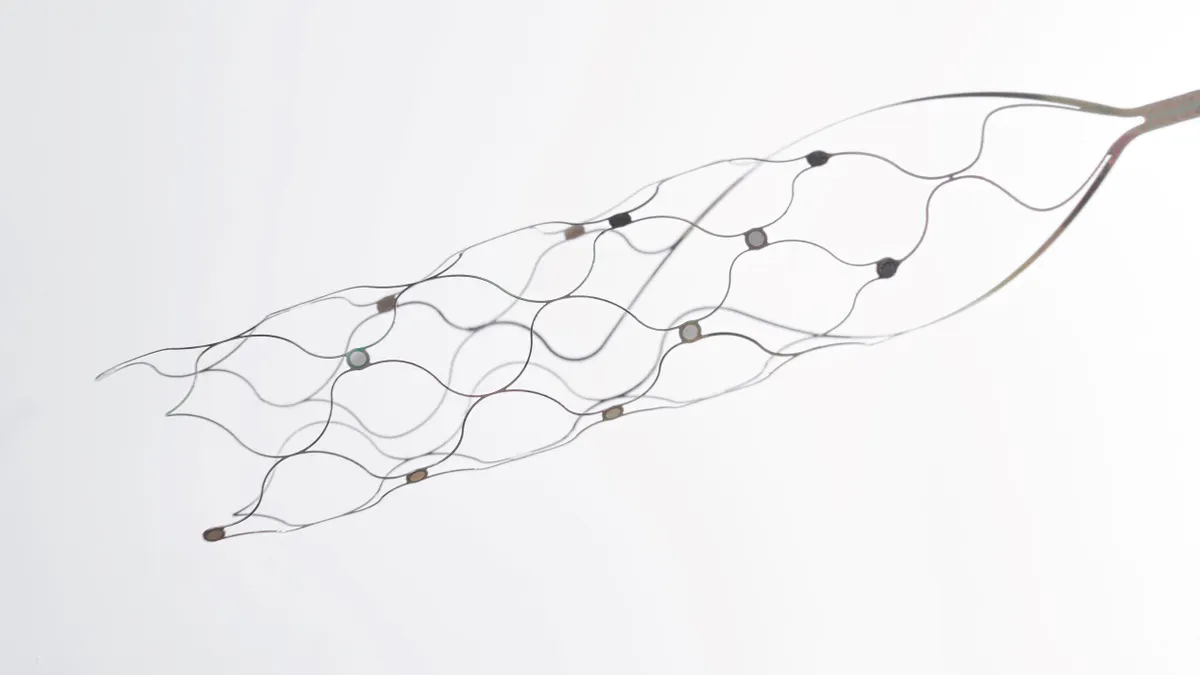
FDA authorizes Pi-Cardia’s valve-in-valve TAVR device
Pi-Cardia designed the Shortcut device to mitigate the risk of coronary artery obstruction by splitting aortic valve leaflets before valve placement.
By Nick Paul Taylor • Oct. 3, 2024 -
Philips issues fix for ventilator problems linked to 9 injuries, 1 death
Philips is asking customers to immediately install a software update to address several new and previously reported safety issues, the FDA said.
By Elise Reuter • Oct. 2, 2024 -
Integer sells non-medical business for $50M
After making nearly $550 million worth of acquisitions in the past few years, the medical device contract manufacturer will use proceeds from the sale to pay down debt.
By Susan Kelly • Oct. 2, 2024 -
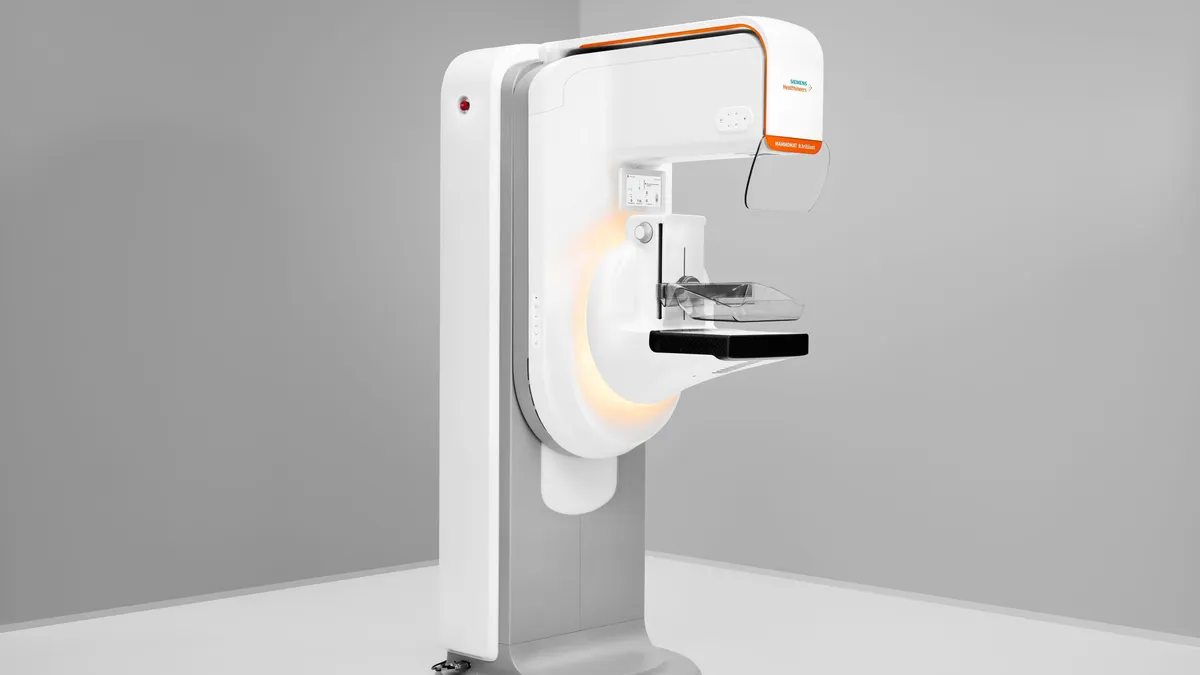
Siemens Healthineers wins FDA approval for 3D mammography system
The 3D capabilities are part of Siemens’ first completely redesigned mammography platform in over a decade.
By Nick Paul Taylor • Oct. 2, 2024 -
GE Healthcare gets FDA nod for new PET imaging agent
Called Flyrcado, the radiotracer could help improve cardiac imaging accuracy in patients with a high body mass index and women, the company said.
By Susan Kelly • Oct. 1, 2024 -
Top medtech conferences in 2025
The lineup includes industrywide conferences and events covering the latest medtech trends in diabetes, orthopedics, cardiac care and surgical robotics.
By Ricky Zipp • Updated Jan. 23, 2025 -
Synchron’s brain-computer interface tech meets safety goal
The six-patient trial also indicated the device worked, according to Synchron, with the conversion of brain signals to motor outputs allowing people to perform digital tasks.
By Nick Paul Taylor • Oct. 1, 2024 -
Baxter closes dialysis solutions facility due to Hurricane Helene damage
More than 2,500 people work at the facility, which is Baxter’s largest manufacturing site. The company is still working to fully assess the damage.
By Elise Reuter • Updated Sept. 30, 2024 -
What’s next at Masimo after CEO Kiani’s exit?
Investors are waiting to learn the next steps for the patient monitoring company after a hard-fought proxy battle, including how a separation of its consumer business could play out.
By Susan Kelly • Sept. 30, 2024 -
Establishment Labs wins FDA approval for Motiva breast implants
Motiva is the first new breast implant to receive premarket approval since 2013, according to Establishment Labs, and enters a market reshaped by safety issues linked to other products.
By Nick Paul Taylor • Sept. 30, 2024