Medical Devices: Page 125
-
5-year Edwards results: TAVR matches surgery on survival, but repeat hospitalizations more common
Separately, a pair of cardiologists in an editorial published this week in the journal Circulation noted potential "important differences" between Edwards' Sapien 3 and Medtronic’s CoreValve devices.
By Nick Paul Taylor , Maria Rachal • Jan. 30, 2020 -
Major conglomerates 3M, GE, Philips bet on healthcare
The corporate giants' earnings reports this week indicate medical segments are increasingly important to strategy as other businesses decline.
By Nick Paul Taylor • Jan. 30, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
UK moves to mitigate impact of notified body withdrawals
Several firms pulled their services amid uncertainty about the impact of Brexit, bringing risks into focus.
By Nick Paul Taylor • Jan. 30, 2020 -
Robot sales spike to new high as Stryker eyes growth in Japan
As Stryker keeps integrating K2M into its spine business and works to close the upper extremities-driven Wright Medical buyout in 2020, momentum with Mako robot sales is helping orthopaedics share.
By Maria Rachal • Jan. 29, 2020 -
FDA clears Eko's stethoscope algorithms for AFib, heart murmur screening
The authorization gives primary care physicians an enhanced tool to screen for serious heart conditions, with the aim of speeding diagnosis and treatment.
By Susan Kelly • Jan. 29, 2020 -
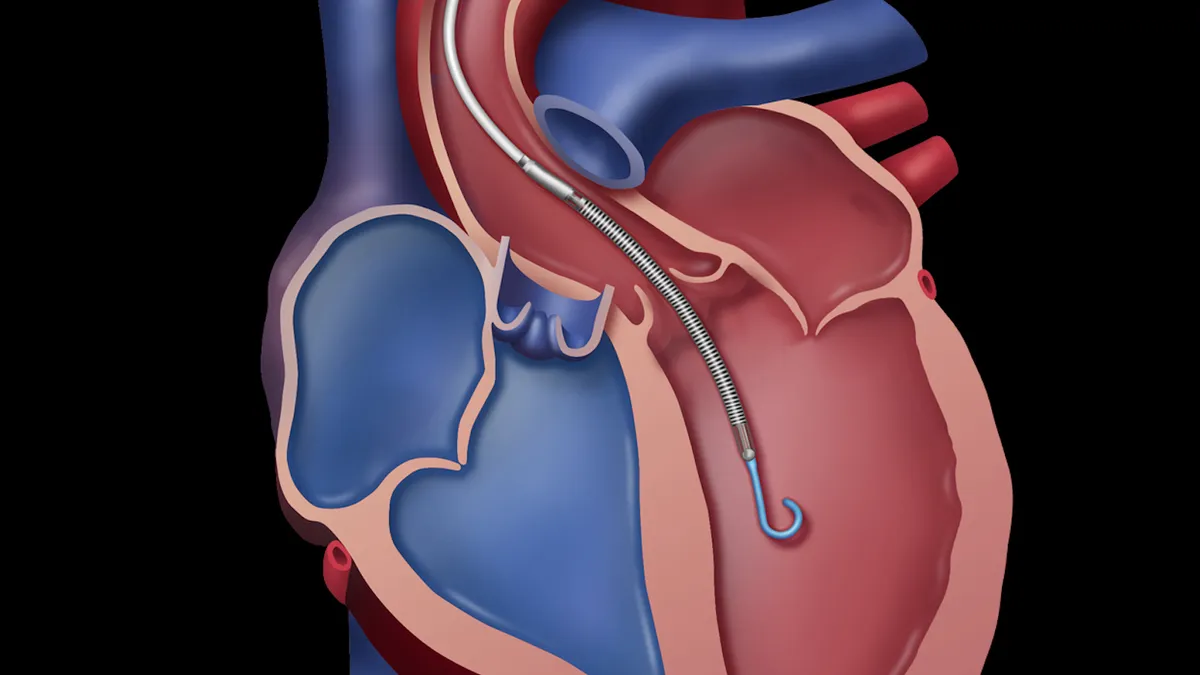
Abiomed device controversy revived in Circulation editorial
Experts flagged key limitations in a study suggesting the Impella device is pricier and more dangerous than intra-aortic balloon pumps, and highlighted an "urgent need" for randomized clinical trials.
By Nick Paul Taylor • Jan. 29, 2020 -
Philips and ResMed may lose share in growing market for sleep tech: survey
Four dozen home medical equipment companies surveyed by analysts at Needham expect sleep patient volumes to grow 15% in the next 12 months, but market leaders may face pressure from smaller competitors.
By Nick Paul Taylor • Jan. 28, 2020 -
After subdued year for new spinal cord stim tech, industry seeks 2020 rebound
Medtronic, Abbott, Boston Scientific and others that presented at the North American Neuromodulation Society meeting hope an influx in new products will help restore market growth.
By Maria Rachal • Jan. 27, 2020 -
FDA offers 510(k) advice for arthroscopy pump tubing to prevent cross-patient infections
In a draft guidance on the irrigation tubing used across minimally invasive joint procedures, the regulator said without proper design, testing and risk mitigation, backflow of patient fluid can present risk for disease transmission.
By Maria Rachal • Jan. 27, 2020 -
Smith & Nephew acquires Tusker's PMA-winning ear infection device for ENT portfolio
The buyout of Tusker Medical comes two months after it won FDA premarket approval for an ear tube delivery system that can be used in young children with local anesthesia alone in a doctor's office.
By Maria Rachal • Jan. 24, 2020 -
Intuitive eyes new surgery types as competitive pressure looms
As noise from robotic surgery newcomers grows, Intuitive is prioritizing wider use of da Vinci in hernia repairs, colorectal procedures and bariatrics.
By Maria Rachal • Jan. 24, 2020 -
Moody's predicts cardiology tech to outpace rest of medical devices, driven by TAVR
The rating agency's report coincided with a more downbeat near-term forecast by Jefferies analysts, who tracked "a sharp step down in December growth" of transcatheter aortic valve replacement system utilization by hospitals.
By Nick Paul Taylor • Jan. 24, 2020 -
GE cybersecurity flaw gets maximum risk score, triggering rare FDA notice
"An attacker could potentially silence an alarm that is intended to communicate vital information about a patient to health care staff, such as a patient's cardiac status," the agency wrote of an issue with certain tech platforms.
By Nick Paul Taylor • Jan. 24, 2020 -
3 more notified bodies coming soon, EC says as MDR clock ticks down
New designations would move the Commission a step closer to its goal of having 20 notified bodies in place by the end of the first quarter of 2020.
By Nick Paul Taylor • Jan. 23, 2020 -
Surgical gown recall sets Cardinal back $96M
The company estimates a $96 million second quarter charge related to the recall, amid two new field actions affecting procedure kits with the gowns. Cardinal previously saw red flags with the supplier at the root of the problem.
By Nick Paul Taylor • Updated Jan. 31, 2020 -
Abbott ups 2020 growth targets, downplays Libre 2 delay
After exceeding the high end of its 2019 sales forecast on success in electrophysiology, heart failure, structural heart and diabetes, the company forecasts up to an 8% rise in organic growth this year.
By Maria Rachal • Jan. 22, 2020 -
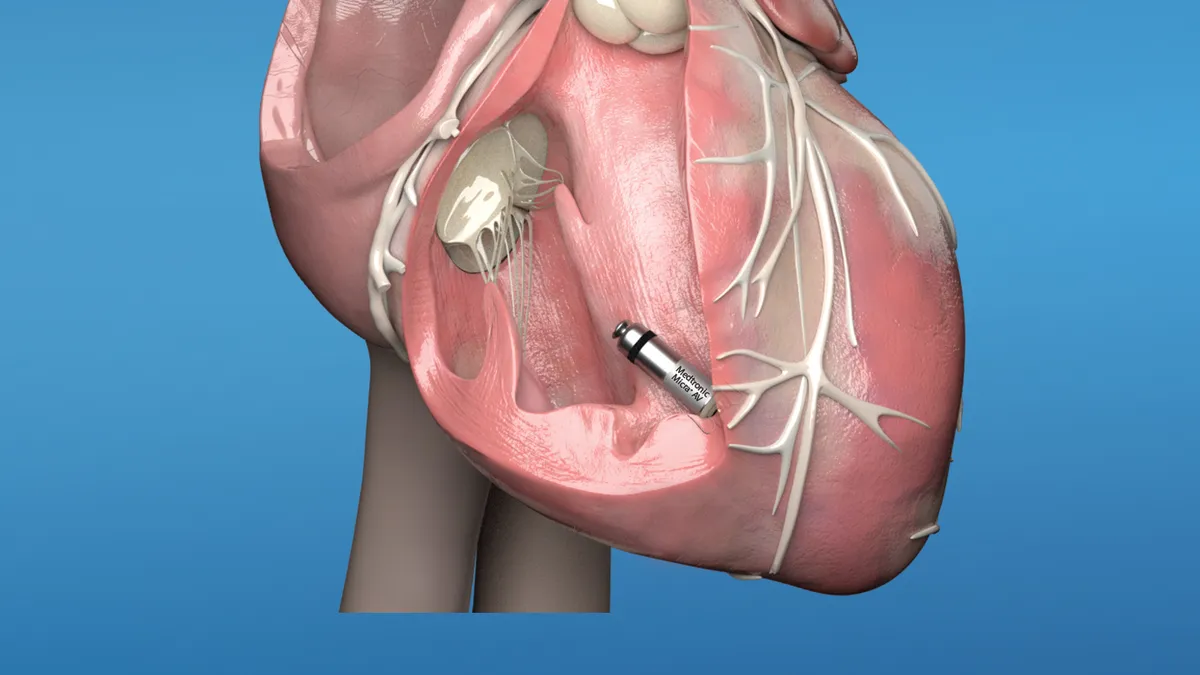
FDA OKs latest Medtronic leadless pacemaker, potentially tripling market
The newest version of the company's transcatheter pacing system, first approved in 2016, expands the device to patients with atrioventricular block.
By Susan Kelly • Jan. 22, 2020 -
Medical device sales flat, J&J CEO promises big year for robots
A Medical Device Business Review Day scheduled for this spring in New York City will feature "tangible evidence of not only the machines, but also the digital platform component," Alex Gorsky said of J&J's robotic surgery ventures.
By Nick Paul Taylor • Updated Jan. 23, 2020 -
HHS price transparency efforts may decode only sliver of total spending
A new analysis focused on non-emergency care that a patient can choose deliberately, such as hip or knee replacements, as opposed to emergency services.
By Ron Shinkman • Jan. 21, 2020 -
Medline's Illinois sterilization plant comes back online
Having spent $10 million to meet stricter ethylene oxide emissions rules, the facility resumed operations Friday, roughly two months after local health regulators once predicted.
By Maria Rachal • Updated March 30, 2020 -
UK health authority 'proactively' monitoring breast implant illness reports
In guidance for patients and clinicians on symptoms "sometimes referred to as Breast Implant Illness," the Medicines and Healthcare products Regulatory Agency stopped short of linking the devices to reported illnesses.
By Susan Kelly • Jan. 21, 2020 -
5 medtech trends to track in 2020
With the medical device tax in the rearview mirror, industry worries include the uncertain future for product sterilization, the "valley of death" between product authorization and reimbursement, and enforcement of new EU regulations.
By Maria Rachal , David Lim • Jan. 17, 2020 -
Virtual, augmented reality in medicine pique FDA's interest
The agency is hosting a workshop Wednesday with representatives from Microsoft, Facebook, Philips and other industry players in hopes of identifying roadblocks to developing more uses of extended reality for medical purposes.
By Nick Paul Taylor • Jan. 17, 2020 -
Edwards' US patent challenge to Abbott MitraClip IP denied
In a decision Thursday, the Patent Trial and Appeal Board thwarted Edwards Lifesciences' petition arguing certain Abbott mitral valve repair claims are unpatentable, the latest in a transatlantic battle between the companies.
By Nick Paul Taylor • Jan. 17, 2020 -
Dexcom talks up Type 2 market, Medtronic says it has 'work to do' in diabetes at JPM
The San Francisco conference featured presentations from most major insulin delivery and glucose monitoring players, preceded by a handful of new funding announcements including a $93 million Series C for Virta Health.
By Maria Rachal • Jan. 16, 2020