Medical Devices: Page 124
-
FDA tracks uptick in masks, other protective gear in coronavirus update
Commissioner Stephen Hahn said the shift in ordering patterns is yet to manifest in a shortage but warned Friday the situation is “evolving and very dynamic.”
By Nick Paul Taylor • Feb. 18, 2020 -
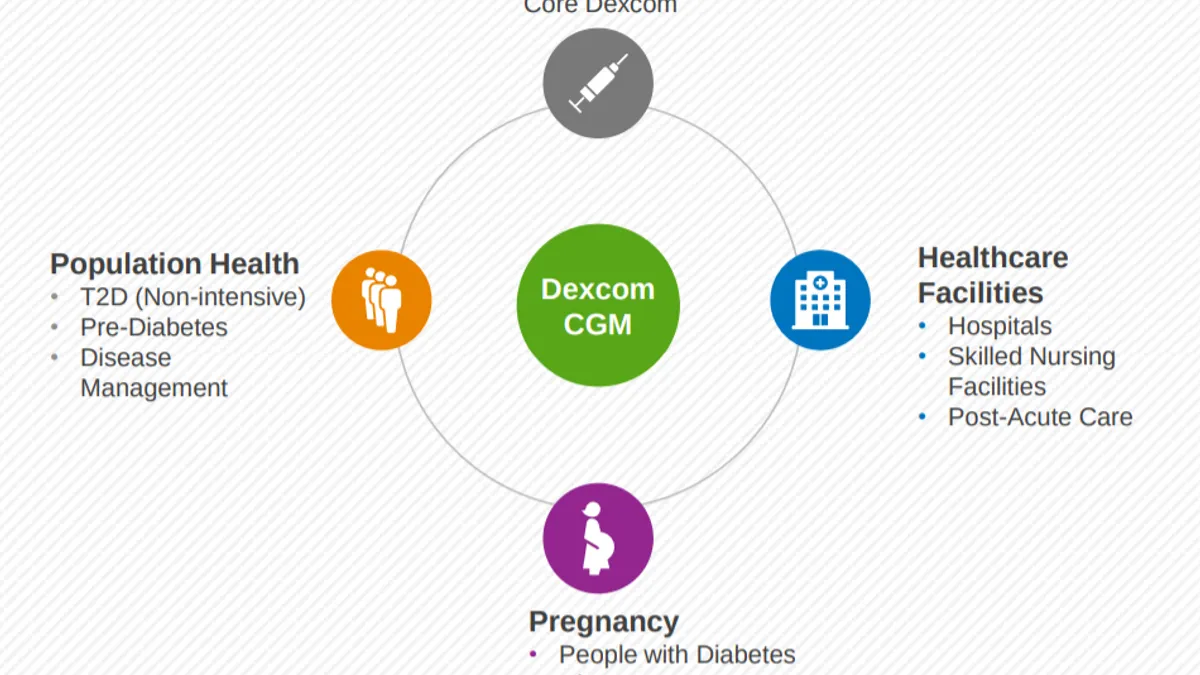
Dexcom hopes wave of new customers offsets lower prices, Abbott's sweet spot
The continuous glucose monitor maker also said it's planning a third manufacturing site, this time outside of the U.S., as it prepares for demand beyond its mainstay Type 1 diabetes market.
By Maria Rachal • Feb. 14, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Baxter overstated income by $278M, internal probe finds
The fallout may drag on from Baxter's use of a flawed method to calculate exchange rates over several years. The medtech continues to work with the SEC and potential investor class action lawsuits could follow.
By Nick Paul Taylor • Feb. 14, 2020 -
Insulet warns of defect with Dash personal diabetes manager
The notice came a day after FDA flagged a potential safety issue with certain Medtronic insulin pumps. Although the source of risk was different, the possible outcome was the same: under or over delivery of insulin.
By Maria Rachal • Feb. 13, 2020 -
Moody's: Coronavirus spread in US to have mixed credit effects across healthcare
Device makers — particularly Boston Scientific, BD and Abbott — will likely see a credit negative effect from supply chain disruption, even if the outbreak is contained to China.
By Shannon Muchmore • Feb. 13, 2020 -
FDA folds Cook Medical asks into final peripheral vascular atherectomy device 510(k) guidance
The agency made some changes to advice on premarket submissions for technologies used to help remove plaque from diseased arteries.
By Nick Paul Taylor • Feb. 13, 2020 -
Medtronic insulin pump recall tied to 1 death, more than 2K injuries, FDA says
A hardware issue the medtech giant flagged to certain MiniMed customers in November has generated more than 26,000 complaints, the agency said as it classified the recall in its highest-risk category.
By Maria Rachal • Feb. 12, 2020 -
Tenet to pay $1.4M to settle claims it unnecessarily implanted cardiac monitors
The unneeded procedures subjected patients to "needless peril, and taxpayers end up with the bill," said an HHS Office of Inspector General special agent.
By Samantha Liss • Feb. 12, 2020 -
FDA OKs 'Inside-Out' device for enabling hemodialysis in hard-to-treat patients
Bluegrass Vascular Technologies plans to make its De Novo-winning venous access device available at select U.S. sites in the coming months, adding to the revenue it already generates in the EU, Canada and other markets.
By Nick Paul Taylor • Feb. 12, 2020 -
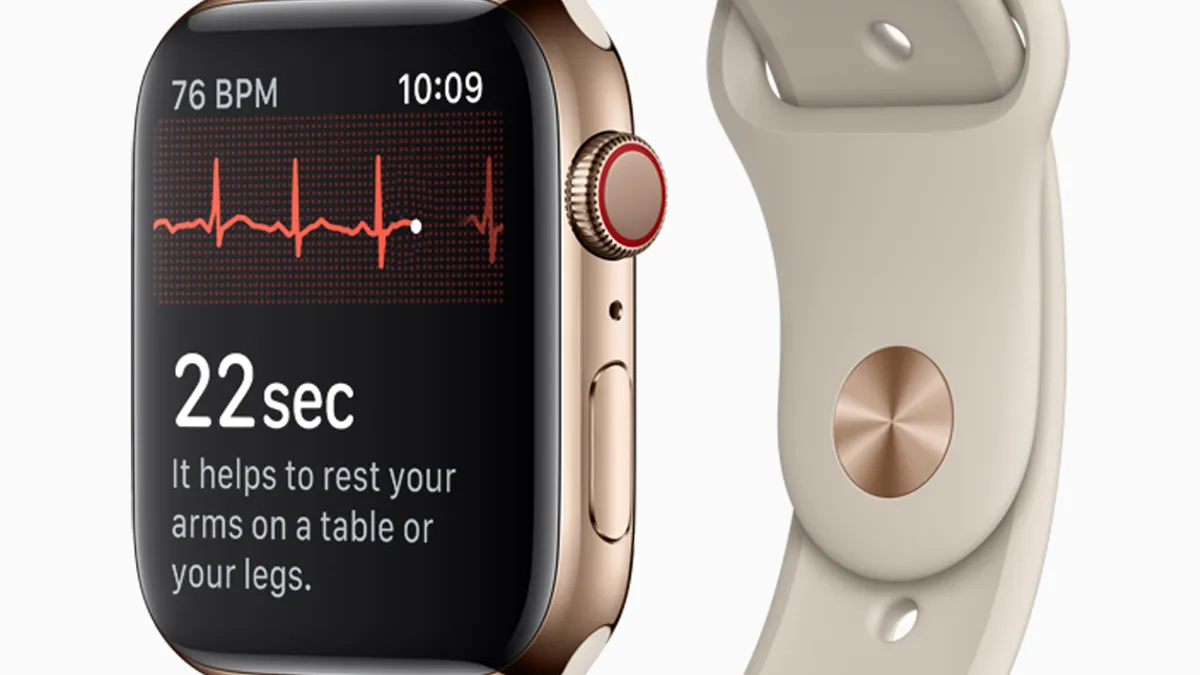
Wearable heart rate sensor accuracy didn't vary across skin tones in small Duke study
But data from 53 individuals, published Monday, revealed differences in accuracy by device manufacturer across activity type.
By Susan Kelly • Feb. 11, 2020 -
Hospitals, clinics most likely to be hit with ransomware attack
But medical testing and supplies companies weren't spared among the 172 individual attacks from 2016 to 2019 that affected 6.6 million patients.
By Shannon Muchmore • Feb. 11, 2020 -
Renewed calls for Impella RCT as JAMA details bleeding, death rate findings
Abiomed has cast doubt on the registry-based study, though some cardiologists argue it supports “a more restrictive use” of the circulatory support device.
By Nick Paul Taylor • Feb. 11, 2020 -
Medtronic faces key test in establishing US market for renal denervation
After a setback six years ago, the medtech giant is set to unveil pivotal trial data next month on a procedure to lower blood pressure. Abbott and Boston Scientific are likely watching closely.
By Susan Kelly • Feb. 10, 2020 -
FDA puts high-risk label on GE Healthcare anesthesia systems recall
A potential loose cable connection in more than 3,600 of the devices may result in a loss of mechanical ventilation.
By Maria Rachal • Feb. 10, 2020 -
Snapshot: How medtech earnings season is shaping up
Most medtechs offered financial outlooks for 2020 over the last month, but the novel coronavirus could have a longer impact than originally factored in.
By Maria Rachal • Updated March 6, 2020 -
Abiomed, on defense, plans wave of trials to counter Impella criticism
The maker of the heart pump is seeking to quash doubts after two large observational studies suggested the product was more risky and pricey than alternative treatments.
By Nick Paul Taylor • Feb. 6, 2020 -
EU MDR notified bodies hit 11 with Irish group added
The National Standards Authority of Ireland describes itself as the country's official standards body. It's the second certified since the Commission teased last month that three more of the entities would soon be added to the count.
By Kim Dixon • Updated Feb. 13, 2020 -
BD cuts 2020 guidance on Alaris pump hangup with FDA, stock tanks
"Resetting expectations is certainly not how I want to start my first call as CEO," new BD head Tom Polen told analysts. Shares were down as much as 13% Thursday morning.
By Maria Rachal • Feb. 6, 2020 -
Boston Scientific expects up to $40M hit in Q1 from coronavirus
Ongoing integration of its $4.2 billion acquisition of BTG and pricing headwinds for drug-eluting stents may also temper growth, the company said on its earnings call Wednesday.
By Maria Rachal • Feb. 5, 2020 -
Merck spins off lower growth products, including contraceptive implants
A yet-to-be-named new company will house the pharma's legacy drugs and women's health products NuvaRing and Nexplanon.
By Jacob Bell • Feb. 5, 2020 -
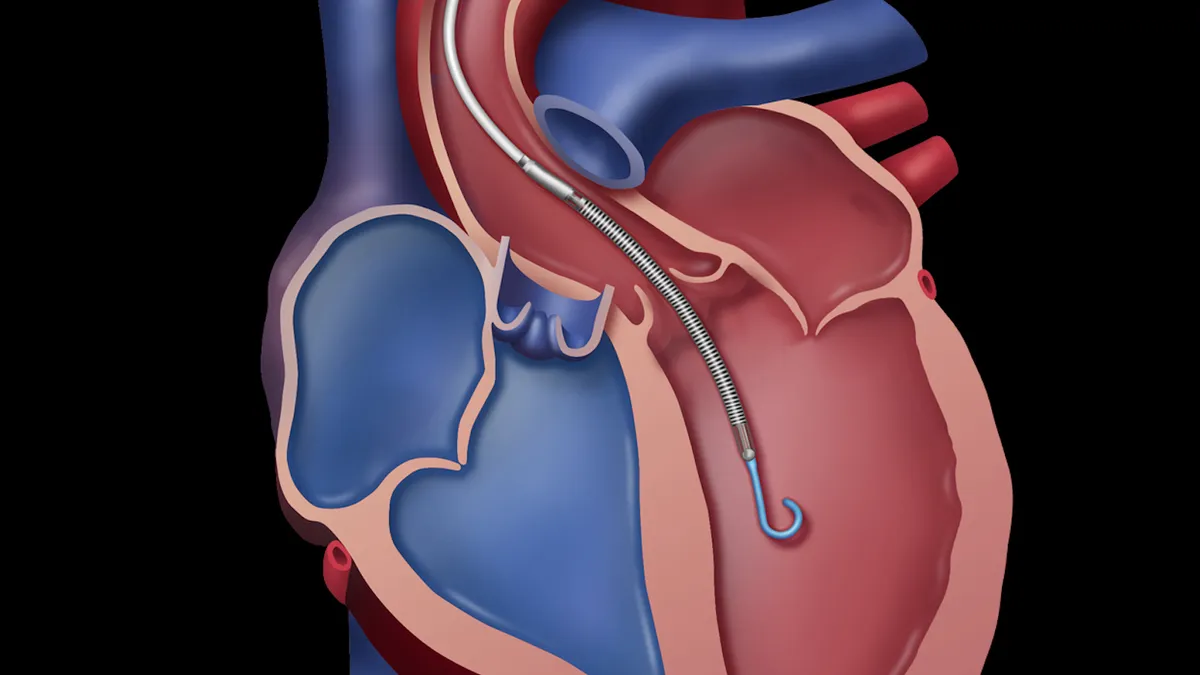
Abbott, SynCardia requests prompt CMS to reconsider heart device coverage
Those companies, plus competitors like Medtronic, stand to benefit if the agency expands access to ventricular assist devices and artificial hearts based on new evidence.
By Nick Paul Taylor • Feb. 5, 2020 -
Abbott wins breakthrough tag for fully implantable LVAD system, following Medtronic
Abbott and Medtronic, whose HeartMate and HeartWare go head-to-head in the left ventricular assist device market, each have FDA's help in getting a less cumbersome version of the treatment to patients with advanced heart failure.
By Maria Rachal • Feb. 4, 2020 -
Zimmer's Rosa robot drives Q4 growth, restructuring set
But the company did not specify total knee system placements like robotics competitor Stryker. It's going to "take a while to disrupt that inertia" from rival systems, CEO Bryan Hanson told investors.
By Maria Rachal • Updated Feb. 4, 2020 -
Contura nabs FDA nod for female incontinence device
The London-based company may compete with Allergan, which also sells a bulking agent.
By Susan Kelly • Feb. 4, 2020 -
Brexit happened. EU MDR looms. 3 key questions on medtech's future in Europe
The U.K. can skip the new in vitro diagnostic rules entirely, and in theory, could unilaterally stop applying MDR after the transition period ends. Both scenarios seem unlikely.
By Nick Paul Taylor • Feb. 4, 2020