Medical Devices: Page 111
-
Philips pens $275M Intact Vascular deal to acquire implant
The buy adds the first FDA-approved vascular implant for below-the-knee interventions to the Dutch conglomerate's portfolio.
By Nick Paul Taylor • Aug. 28, 2020 -
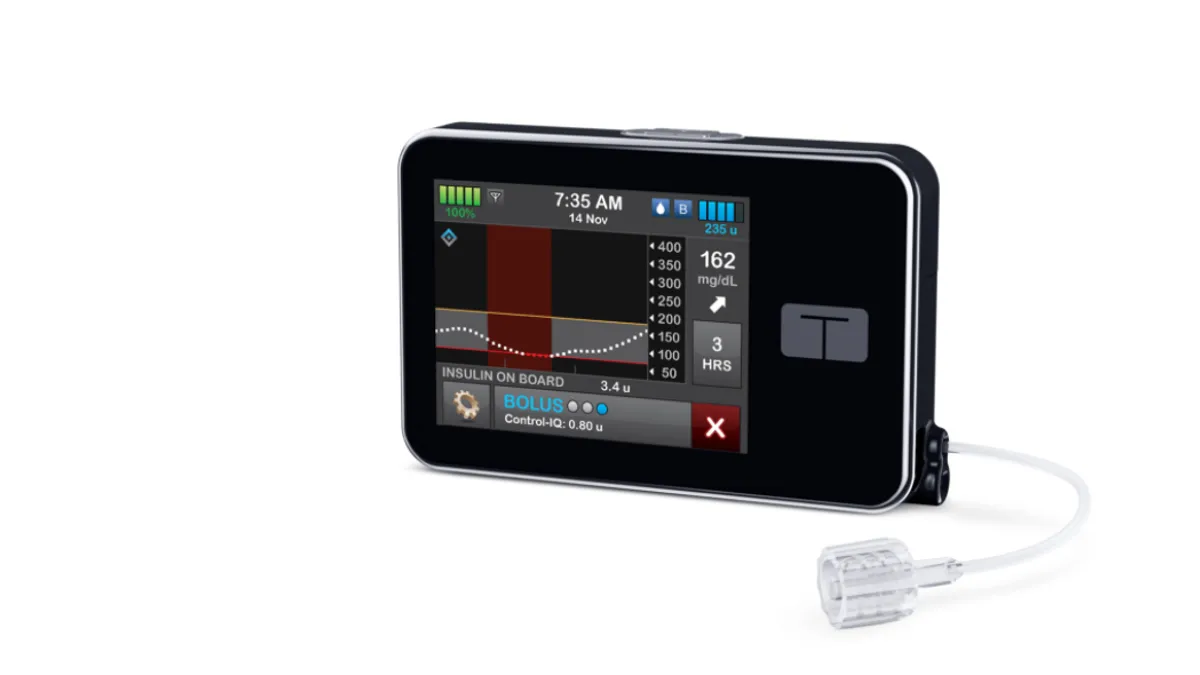
Tandem's artificial pancreas improves glucose control in pediatric trial
Children treated with the closed-loop insulin delivery system spent longer in the target glucose range than peers who just received Dexcom G6 sensors and insulin, the study published in the New England Journal of Medicine found.
By Nick Paul Taylor • Aug. 27, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
With a one-year reprieve to EU's MDR, some procrastinate, others speed ahead
Industry is taking advantage of a long-sought delay to the regulation in different ways, but COVID-19's impact on notified body capacity, pace of information from lawmakers, and company resources are some confounding variables.
By Maria Rachal • Aug. 26, 2020 -
Trump admin delays final rule easing anti-kickback regs until next August
Devicemakers back proposed changes to the law, passed decades ago to deter physicians from referring patients to locations that give them a financial benefit, calling them outdated and burdensome.
By Rebecca Pifer • Aug. 26, 2020 -
Hologic inks $80M Acessa buyout to expand women's health business
The cash acquisition, plus contingent payments based on future revenue growth, will add a treatment for benign uterine fibroid to the medtech's portfolio.
By Nick Paul Taylor • Aug. 26, 2020 -
Insulin pumps among millions of devices facing risk from newly disclosed cyber vulnerability, IBM says
The firm's hacking team said the vulnerability may allow criminals to remotely alter patient dosing, as well as manipulate readings from medical device monitors "to cover up concerning vital signs or create false panic."
By Greg Slabodkin • Aug. 25, 2020 -
Medtronic's Martha teases focus shift on diabetes, robotics
After pandemic-driven delays to its soft tissue robot, the company also told investors it expects to file for a CE mark and a U.S. investigational device exemption in the first quarter of calendar year 2021.
By Maria Rachal • Aug. 25, 2020 -
FDA-industry group touts real-world evidence framework to speed test development
The Medical Device Innovation Consortium, a collaboration between FDA and diagnostics makers like Abbott and Roche, offers a roadmap as COVID-19 accelerates use of newly generated data to update emergency authorizations.
By Susan Kelly • Aug. 25, 2020 -
Q&A
MedTech Europe beats drum for virtual audits, IVDR delay
The one-year delay to the EU Medical Device Regulation has been crucial, but there's been "radio silence" from lawmakers on calls for a similar delay to the In Vitro Diagnostic Regulation, said regulatory lead Oliver Bisazza.
By Maria Rachal • Aug. 24, 2020 -
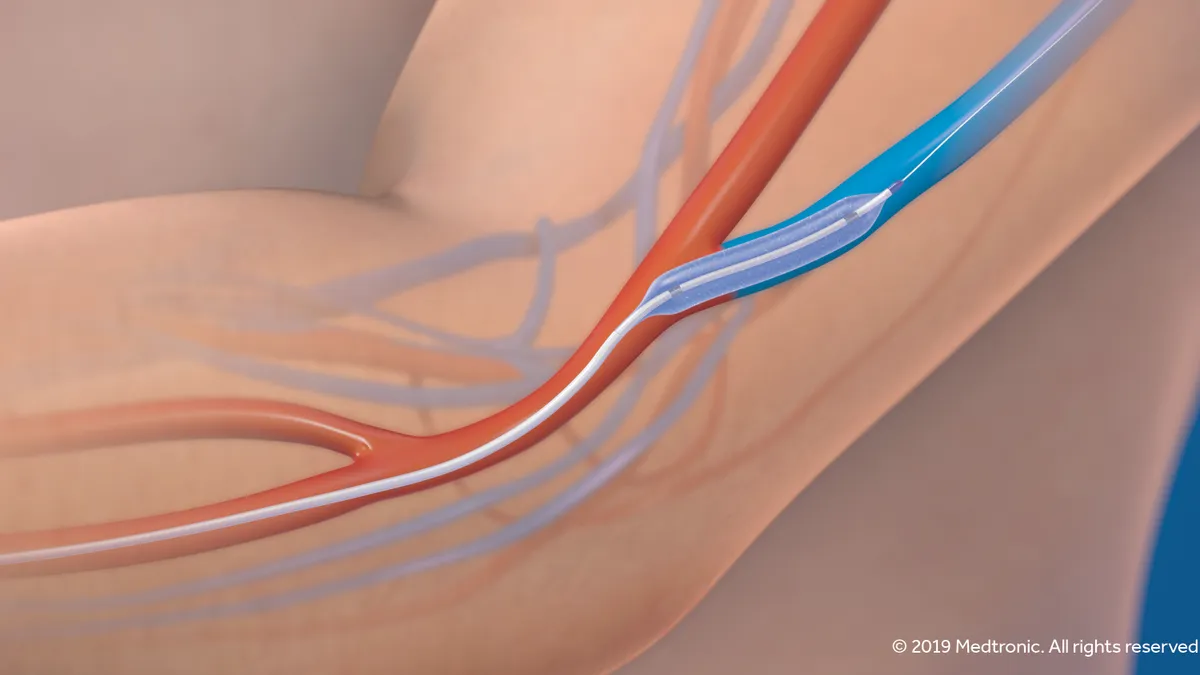
Medtronic hopes for renewed spotlight on dialysis access device with NEJM publication
The company-funded study in the New England Journal of Medicine comes amid widespread disruption of vascular access procedures, and nine months after Medtronic got FDA approval for its IN.PACT AV drug-coated balloon.
By Maria Rachal • Aug. 21, 2020 -
Intuitive robot may up survival in mouth and throat cancers, but not others: study
Researchers in JAMA Oncology said the findings back a randomized clinical trial for oropharyngeal patients. The da Vinci system had no effect on overall survival in patients with prostate, endometrium and cervix cancers.
By Nick Paul Taylor • Aug. 21, 2020 -
Eudamed's first section to go live before year's end
The European Commission is ready to move on the initial module of the new safety and performance database, created as part of the incoming Medical Device Regulation, ahead of a March 2021 deadline.
By Nick Paul Taylor • Aug. 19, 2020 -
CMS urges resumption of essential procedures, organ transplants for ESRD patients
Medicare beneficiaries with end-stage renal disease have 3.5 times heightened risk of COVID-19 infection, according to an HHS analysis of early claims data.
By Maria Rachal • Aug. 18, 2020 -
FEMA pitches voluntary DPA pact to bolster medical supply chain during pandemic
The Defense Production Act allows private companies to enter agreements with the government while avoiding antitrust liability. AdvaMed wants to participate but FEMA has not yet extended an invite.
By Greg Slabodkin • Aug. 18, 2020 -
Medtronic seeks leg up in lagging pen market with Companion Medical buy
As Dexcom and Abbott grow share in the continuous glucose monitoring market and Insulet and Tandem forge on with interoperable insulin pump systems, Medtronic is hoping to jump ahead with a connected pen.
By Maria Rachal • Aug. 17, 2020 -
FDA looks to downclass noninvasive bone growth stimulators, incumbents not on board
The agency made a case for moving the devices to less-rigorous Class II regulation in a proposal opposed by manufacturers with products already on the market such as Orthofix.
By Susan Kelly • Aug. 17, 2020 -
Surgical robots, early cancer tests top CB Insights digital health startup list
The most promising, as aggregated in annual rankings from the firm, have raised over $20 billion in total funding for more than 600 deals with 900-plus investors.
By Hailey Mensik , Maria Rachal • Aug. 14, 2020 -
Hospitals, docs pitch roadmap to keep essential surgeries going even with coronavirus surges
While many medtechs cast the worst of the pandemic's impact in the rearview mirror in recent financial reports, concerns from healthcare industry groups reflect a more nuanced picture.
By Nick Paul Taylor • Aug. 14, 2020 -
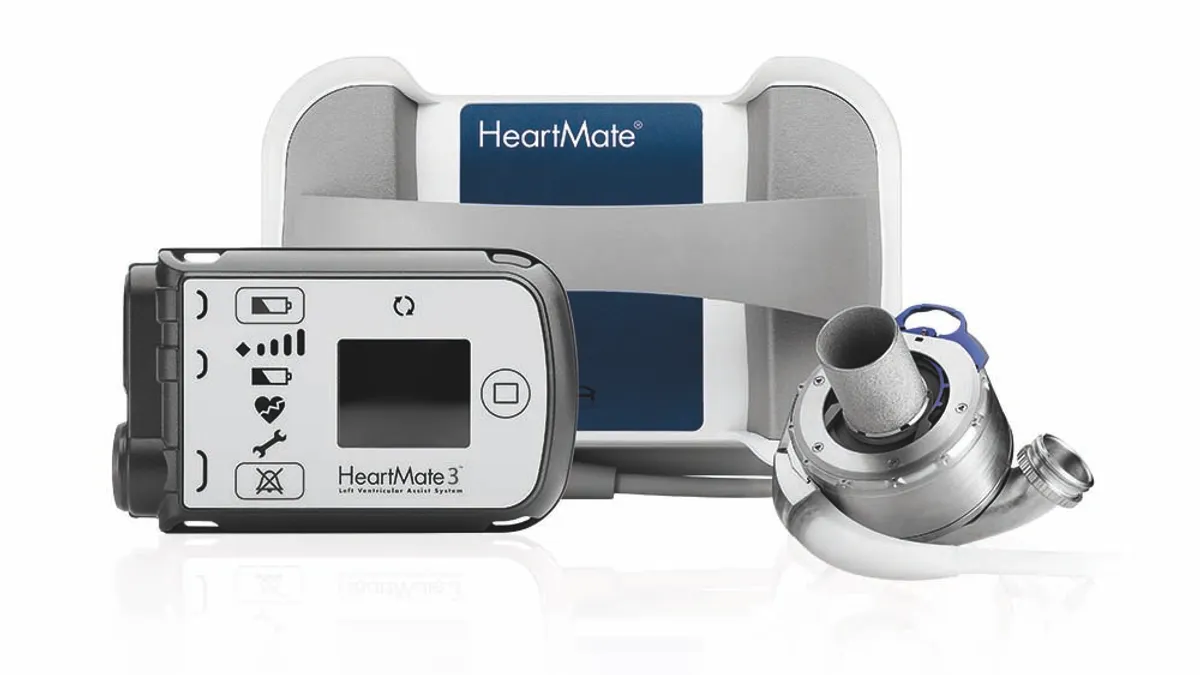
CMS seeks to ease rules on artificial heart, ventricular assist device coverage
Potential eligibility changes for advanced heart failure patients drew positive feedback from Abbott and Medtronic, while removal of a coverage with evidence development policy has been opposed by several cardiologist groups.
By Maria Rachal • Aug. 13, 2020 -
Watchdog again pushes CMS to add UDIs to claim forms
The HHS Office of Inspector General said that without a place to ask for device-specific information, it's difficult for the agency to track billions of dollars in costs related to failed devices.
By Nick Paul Taylor • Aug. 13, 2020 -
Inari Medical revenue rises in 1st quarterly report since public debut
Specializing in clot-removal devices for pulmonary embolism and deep vein thrombosis, the medtech's CEO downplayed new competition in the space, claiming that only 2% of the $3.6 billion market is penetrated.
By Susan Kelly • Aug. 12, 2020 -
Coronavirus muddies FDA's trajectory on device warning letters
An agency official said going into 2020 that its devices center would be better equipped to use the compliance tool in a timely fashion. But a dry spell for on-site surveillance may undermine any noticeable rebound.
By Maria Rachal • Updated Aug. 12, 2020 -
FDA posts Essure adverse events pulled from social media
The spreadsheet includes details of 53 deaths and 1,376 reports of serious injuries related to the controversial birth control implant but the agency is unsure if it contains duplicate reports.
By Nick Paul Taylor • Updated Aug. 14, 2020 -
Medtronic to buy Companion Medical, adding connected insulin pen to diabetes unit
Building on tuck-in acquisitions to a business losing market share in recent years, the deal gives the medtech giant an FDA-cleared device for people with Type 1 or Type 2 diabetes on multiple daily injection therapy.
By Greg Slabodkin • Aug. 11, 2020 -
Sientra claims market gains as breast implant sales rebound faster than rivals
AbbVie's Allergan and Establishment Labs saw deeper declines in second quarter financials. Sientra CEO Jeffrey Nugent boasted the company is "clearly taking share" from rivals and is hiring to meet demand.
By Nick Paul Taylor • Aug. 11, 2020