Medical Devices: Page 110
-
FDA AI-machine learning strategy remains work in progress
Nearly 18 months since the agency's discussion paper on a proposed regulatory framework, critical pieces are still needed for the development of draft guidance, agency digital health chief Bakul Patel said.
By Greg Slabodkin • Sept. 14, 2020 -
FDA advisers support down-classing bone growth stimulators, in face of industry and lawmaker pushback
But the orthopaedic devices panelists said there should be rigorous clinical evidence standards for the class of products currently sold by Orthofix, Zimmer Biomet, Bioventus and DJO, which are yet to be defined.
By Maria Rachal • Sept. 11, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Thermo Fisher hikes growth forecast, expects COVID-19 sales to top $3B in late 2020
The company forecasts significantly higher growth this year than it's posted in the past, despite suffering a slow first quarter due to early disruption from the pandemic.
By Nick Paul Taylor • Sept. 11, 2020 -
FDA aims to harmonize adverse event reporting with eMDR updates, makes patient problem codes public
The agency finalized adding new elements and fields to its Electronic Medical Device Reporting system, and also clarified protocols for manufacturers with EUAs or following COVID-19 enforcement policies.
By Nick Paul Taylor • Sept. 10, 2020 -
The best TAVR device? Experts opine on when to use Boston Scientific, Edwards or Medtronic valve
A JAMA Cardiology review found design differences may give each transcatheter aortic valve replacement device small competitive advantages in certain circumstances.
By Nick Paul Taylor • Sept. 10, 2020 -
Medtronic tricuspid valve replacement device wins FDA breakthrough status
Although the medtech's Intrepid device is just starting an early feasibility study, it may ultimately go up against transcatheter tricuspid valve repair options from Abbott and Edwards.
By Maria Rachal • Sept. 9, 2020 -
Ultrasound ablation provided 'adequate' short-term control for prostate cancer patients, small study shows
Only 9% of men treated with products from SonaCare or EDAP TMS required surgery or radiation over the next two years, but larger studies are needed to better show how well HIFU works, a Journal of Urology publication concluded.
By Nick Paul Taylor • Sept. 9, 2020 -
Ortho device reclassifications, MDUFA reauthorization among FDA's fall meeting lineup
The agency's upcoming slate of virtual medtech meetings kicks off Tuesday with an orthopaedic devices panel that will consider a proposal to downclassify noninvasive bone growth stimulators.
By Susan Kelly • Sept. 8, 2020 -
Surgeries keep rising, buoying Wall Street's year-end medtech outlook
Jefferies reported a fourth consecutive week of growing hospital traffic, while Bank of America is "broadly bullish" on the medical devices sector as 2020 wraps up.
By Greg Slabodkin • Sept. 8, 2020 -
Edwards gets FDA approval for Sapien 3 in new patient population
The PMA expands the transcatheter valve for use in congenital heart defect patients who suffer problems following surgical repair, positioning the device to compete against a range of treatment options.
By Nick Paul Taylor • Sept. 8, 2020 -
Abbott resurrects resorbable scaffold tech for below-the-knee trial
The medtech previously stopped selling the technology amid evidence it increased the risk of major adverse cardiac events, but recent data has revealed a new BTK opportunity.
By Nick Paul Taylor • Sept. 4, 2020 -
FDA flags 428 spinal cord stimulator patient deaths, urges more tests before implant
The agency revealed the figures in a letter to healthcare providers urging simulations prior to permanently implanting the devices, sold by companies such as Abbott, Boston Scientific, Medtronic and Nevro.
By Nick Paul Taylor • Sept. 4, 2020 -
Watchdog gives high marks to CMS competitive bidding program
The Office of Inspector General probe found the agency followed procedures 97% of the time when awarding contracts for durable medical equipment, prosthetics, orthotics and supplies.
By Nick Paul Taylor • Sept. 3, 2020 -
Boston Scientific, Stryker win CMS new tech add-on pay, BD left out
When combining eligible devices with other recipients, including drug therapies, the agency estimates spending on the payments will total roughly $874 million, a 120% year-over-year increase.
By Maria Rachal • Sept. 2, 2020 -
Stryker reaches agreement with Colfax for Wright Medical deal divestitures
The medtech disclosed Thursday the orthopaedics competitor's DJO Global subsidiary agreed to acquire its ankle and finger joint replacement businesses to shore up U.S. and U.K. antitrust concerns.
By Maria Rachal • Updated Oct. 16, 2020 -
HHS terminates Hamilton, Vyaire ventilator contracts
The Strategic National Stockpile now has an adequate supply of the devices, the agency said. The two companies will not deliver the additional 38,000 ventilators they'd planned by year's end.
By Greg Slabodkin • Sept. 2, 2020 -
UK outlines post-Brexit medical device regime starting January
Regulators unveiled a future U.K. Conformity Assessed (UKCA) mark, but will continue recognizing CE marks until mid-2023 and give companies four to 12 months to register their devices and in vitro diagnostics.
By Nick Paul Taylor • Sept. 2, 2020 -
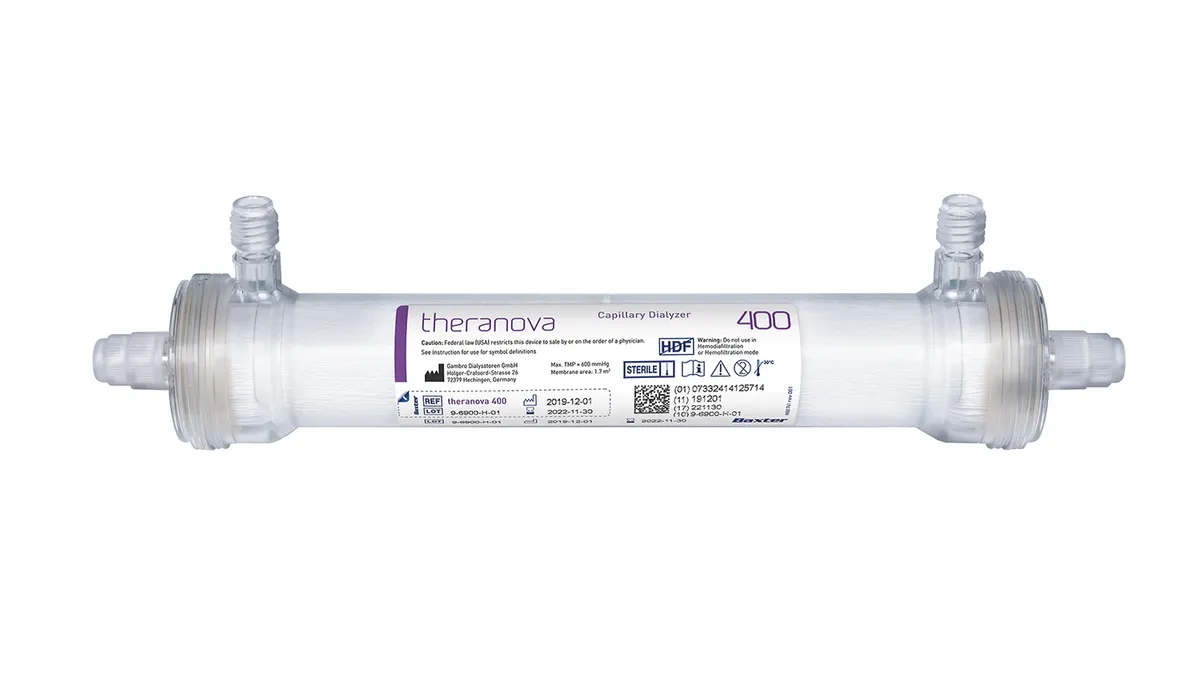
Baxter wins FDA De Novo for more expansive dialyzer
The medtech is still working on convincing CMS to support add-on Medicare payments for the devices.
By Maria Rachal • Sept. 1, 2020 -
Medtronic artificial pancreas gets FDA nod for young kids
The medtech is introducing MiniMed 770G, a Bluetooth-enabled version of the first hybrid closed looped system to get agency approval, bridging the gap to a device launching in Europe this fall but delayed in the U.S.
By Nick Paul Taylor • Sept. 1, 2020 -
CMS pitches coverage of breakthrough devices in tandem with FDA authorization
The proposal follows years of AdvaMed lobbying for products awarded the special FDA designation to gain Medicare reimbursement upon clearance or approval.
By Maria Rachal , Susan Kelly • Aug. 31, 2020 -
FDA proposes adding patient voice in device evaluation
The draft guidance comes nearly two years after an advisory committee prioritized greater inclusion of the patient perspective in device design, development and assessment. Two more public meetings are planned.
By Susan Kelly • Aug. 31, 2020 -
Philips lowers 2020 outlook as HHS nixes ventilator contract
The medtech received a "partial termination" notice and will not deliver 30,700 remaining ventilators to the Strategic National Stockpile, a month after a House panel called for a probe into the roughly $647 million contract.
By Greg Slabodkin • Aug. 31, 2020 -
EU MDR costing smaller medtechs 5% of their annual sales: survey
Almost 70% of 101 companies surveyed by a German software provider said they spend most of their time related to the new rules deciphering what the incoming EU Medical Device Regulation means.
By Nick Paul Taylor • Aug. 31, 2020 -
Stryker stretches Wright Medical share offer into Q4
As the medtech makes the fifth offer extension since the $4 billion acquisition was announced last November, the company is approaching the latest time it said the deal would close.
By Susan Kelly • Updated Sept. 29, 2020 -
Clinical trial links hospital use of Dexcom G6 to improved glucose control
Scripps Whittier Diabetes Institute researchers found the sensors safe and effective in the hospital setting, newly cleared by FDA amid the pandemic. Whether the benefits justify the additional cost is unclear.
By Nick Paul Taylor • Aug. 28, 2020