Recalls: Page 3
-
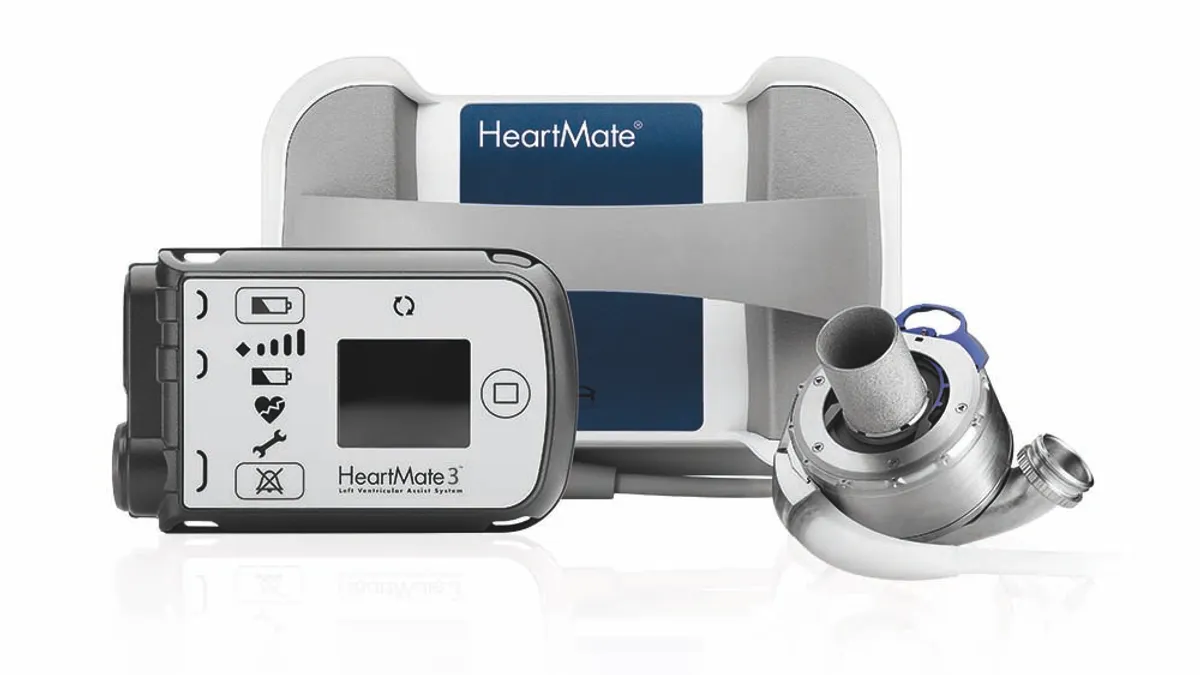
Abbott issues recall for Heartmate system monitor
Abbott’s heart pump system and related products have been part of multiple Class I recalls this year.
By Susan Kelly • June 11, 2024 -
Medtronic recalls neurosurgery navigation system for software error
A problem with the Stealthstation S8 could cause incorrect device placement during surgery. It is the fourth recall of Stealthstation software in the past 12 months.
By Elise Reuter • June 6, 2024 -
Philips reports 7 deaths linked with BiPAP alarm problem
Philips recalled three BiPAP models because an alarm can incorrectly engage and shut down the ventilator under certain circumstances.
By Elise Reuter • May 29, 2024 -
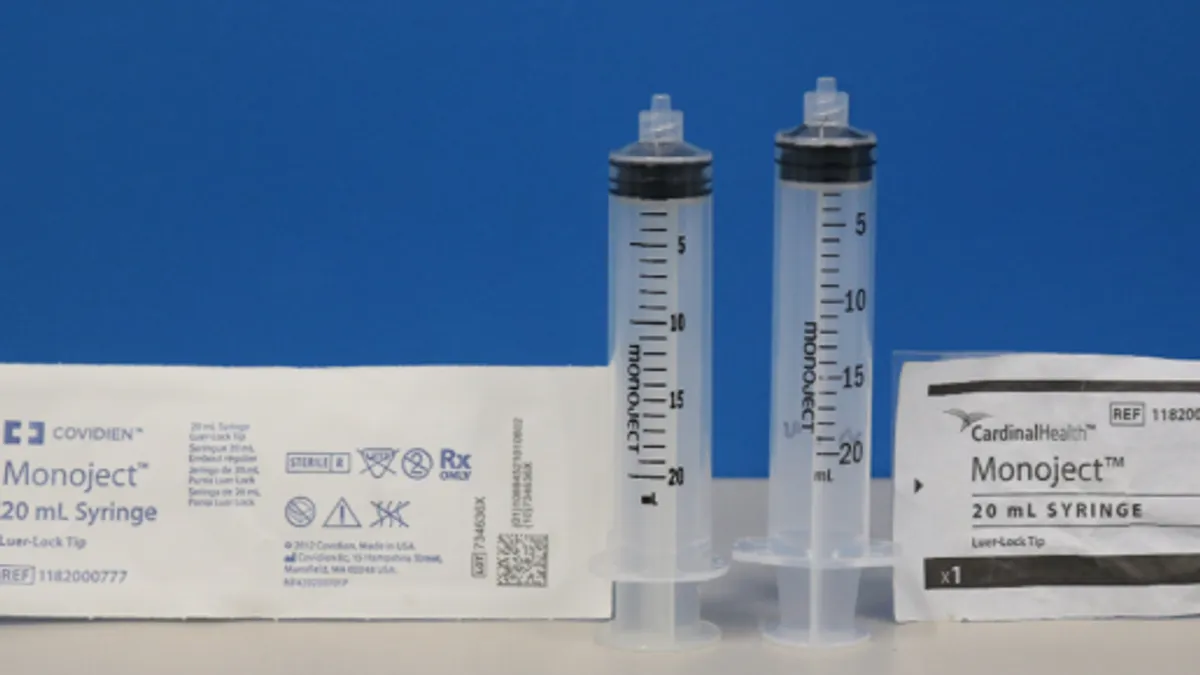
Medline, Chinese manufacturer launch recalls for plastic syringes
Medline and Jiangsu Shenli Medical Production are at the center of the FDA’s probe of plastic syringes made in China.
By Ricky Zipp • May 24, 2024 -

 Retrieved from Hologic on May 23, 2024
Retrieved from Hologic on May 23, 2024
Hologic recalls more than 53,000 radiographic markers linked to 71 injuries
The Biozorb devices are staying on the market, but Hologic has asked people to report adverse events such as pain.
By Nick Paul Taylor • May 23, 2024 -
Boston Scientific recalls more than 1M angiographic catheters
Hospitals should discontinue use of the devices and return them due to potential safety risks, Boston Scientific wrote in a March letter to customers.
By Susan Kelly • May 15, 2024 -
Abbott recalls Heartmate pump after 70 injuries, 2 deaths reported
In the latest notice, which follows a February recall of the device for a separate issue, Abbott took action due to reports of blood leakage and air entrapment.
By Susan Kelly • Updated May 16, 2024 -
J&J’s Megadyne discontinues pediatric electrode pad after burn reports
Megadyne discontinued the product globally after receiving four reports of patients being burned in procedures when the electrode pads were used.
By Nick Paul Taylor • May 13, 2024 -
Route 92 catheter recall tied to 2 injuries, 1 death
The affected devices were manufactured by an outside supplier, not Route 92, and had product quality problems.
By Nick Paul Taylor • May 13, 2024 -
Tandem reports 224 injuries related to faulty insulin pump app
A problem with Tandem’s iPhone app could drain the battery of the connected insulin pump, causing it to power down sooner than expected.
By Nick Paul Taylor • May 9, 2024 -
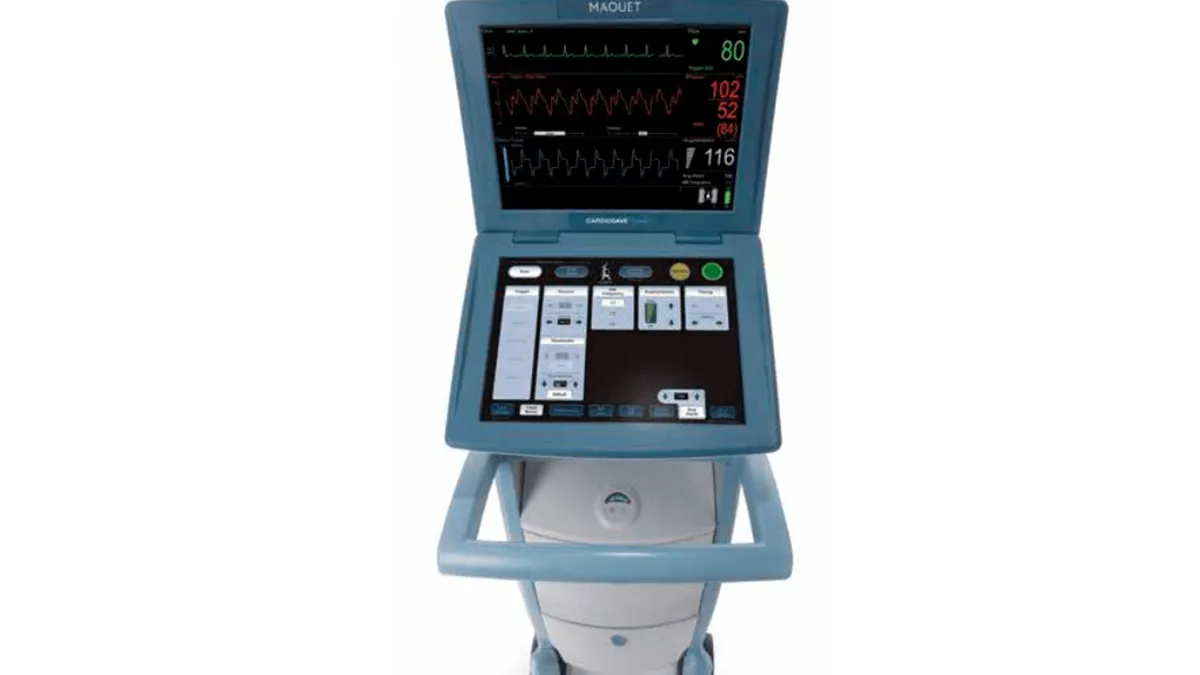
FDA tells providers to stop using Getinge heart devices
After months of safety concerns, the FDA said Getinge has yet to “sufficiently” address the problems and risks.
By Nick Paul Taylor • May 9, 2024 -
 Retrieved from Food and Drug Administration.
Retrieved from Food and Drug Administration.
Cardinal receives FDA warning letter over unapproved syringes
The agency has recently increased scrutiny of plastic syringes made in China because of safety concerns, including blocking imports from two Chinese manufacturers.
By Elise Reuter • May 2, 2024 -
Philips reaches major settlements related to respiratory devices recall
A judge recently finalized an economic claims settlement, and Philips said it reached an agreement on medical claims.
By Elise Reuter • May 2, 2024 -
Philips agrees to $1.1B settlement of US respiratory recall lawsuits
CEO Roy Jakobs said on a first-quarter earnings call that “for the U.S., this is as final as it can get.”
By Nick Paul Taylor • April 29, 2024 -
Fresenius Medical Care recalls 2M dialysis devices over toxin exposure risk
Nearly 2.2 million devices are affected by the recall. Fresenius Medical Care will not have to remove products due to the issue.
By Nick Paul Taylor • April 26, 2024 -
Infutronix infusion pump recall linked to 6 injuries, 1 death
The FDA’s recall notice sheds light on the impact to patients weeks after the company told customers it would pull Nimbus pumps from the market.
By Nick Paul Taylor • April 26, 2024 -
Judge approves settlement of more than $500M from Philips recall
Philips could be on the hook for more costs if eligible claims for recalled or returned respiratory devices exceed the amount agreed to in the settlement.
By Elise Reuter • April 25, 2024 -
Philips failed to report corrections of CT machines, FDA says in warning letter
After a 2023 inspection, the FDA found three unreported field corrections related to CT machines and 19 other recalls of radiology devices.
By Elise Reuter • April 25, 2024 -
Exactech recalls shoulder devices after initially declining to act
After the FDA issued a public safety notice and sent Exactech a warning letter, the firm agreed to recall shoulder implants due to packaging issues.
By Nick Paul Taylor • April 22, 2024 -
Boston Scientific recalls blood-blocking agent linked to 2 deaths
Seven injuries and 11 incidents were also associated with the safety issue.
By Nick Paul Taylor • April 18, 2024 -
Abbott’s latest Heartmate recall tied to 273 injuries, 14 deaths
Biological material can build up and obstruct blood flow in heart failure patients supported by the left ventricular assist devices, the FDA said.
By Susan Kelly • April 15, 2024 -
Philips restricted from selling respiratory machines in DOJ consent decree
The agreement, filed Tuesday, also has export restrictions to ensure U.S. patients receive remediated devices in a timely manner.
By Elise Reuter • April 9, 2024 -
Smiths Medical recalls thousands of ventilators over fault linked to 8 serious injuries
A problem with the devices can cause patients to receive the wrong amount of ventilation or too little oxygen, the FDA said in a recall notice.
By Nick Paul Taylor • April 5, 2024 -
Teleflex catheterization kit recall linked to 10 injuries, 1 death
Nearly 335,000 devices were recalled due to safety issues, which may cause damage to blood vessel walls, artery blockage or death.
By Nick Paul Taylor • April 4, 2024 -
Infutronix pulls infusion pumps from US after nearly 3,700 complaints
The company listed six issues that can cause the pumps to stop infusions and otherwise malfunction.
By Nick Paul Taylor • April 2, 2024