FDA: Page 9
-
FDA partners with Gates Foundation to support breath-based diagnostic development
Through a $1.9 million Gates Foundation grant, the collaborators will create methods to help developers make tests that can easily be deployed to rural and remote areas.
By Nick Paul Taylor • June 27, 2024 -
Cardinal Health recalls procedure kits over plastic syringes made in China
Cardinal listed more than 500 catalog numbers in its recall notice and told customers to remove and discard the syringes.
By Nick Paul Taylor • June 24, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Q&A
ONC’s Micky Tripathi on laying the digital floor for healthcare AI
The agency head discussed ONC’s accomplishments over the past two decades, artificial intelligence opportunities and improving the documentation burden among clinicians.
By Emily Olsen • June 20, 2024 -
FDA official sets out approach to AI in medical devices
Developing quality assurance practices for AI models should be a priority, said Troy Tazbaz, director of the CDRH’s Digital Health Center of Excellence.
By Elise Reuter • June 18, 2024 -
Senators urge CMS to create AI payment pathway
Uniform reimbursement standards are needed to protect patient access to algorithm-based devices and ensure future innovation, the lawmakers said.
By Nick Paul Taylor • June 18, 2024 -
FDA creates transparency principles for AI in medical devices
The agency worked with regulators in the U.K. and Canada to determine how key information about machine learning-enabled devices should be communicated.
By Nick Paul Taylor • June 17, 2024 -
Medtech group updates EtO standard for first time in 25 years
The Association for the Advancement of Medical Instrumentation said the changes incorporate new technologies and address industry challenges.
By Elise Reuter • June 14, 2024 -
Teleflex catheter kit recall linked to 31 injuries, 3 deaths
Teleflex reported 322 complaints about a safety problem that can prevent the balloon from fully inflating.
By Nick Paul Taylor • June 14, 2024 -
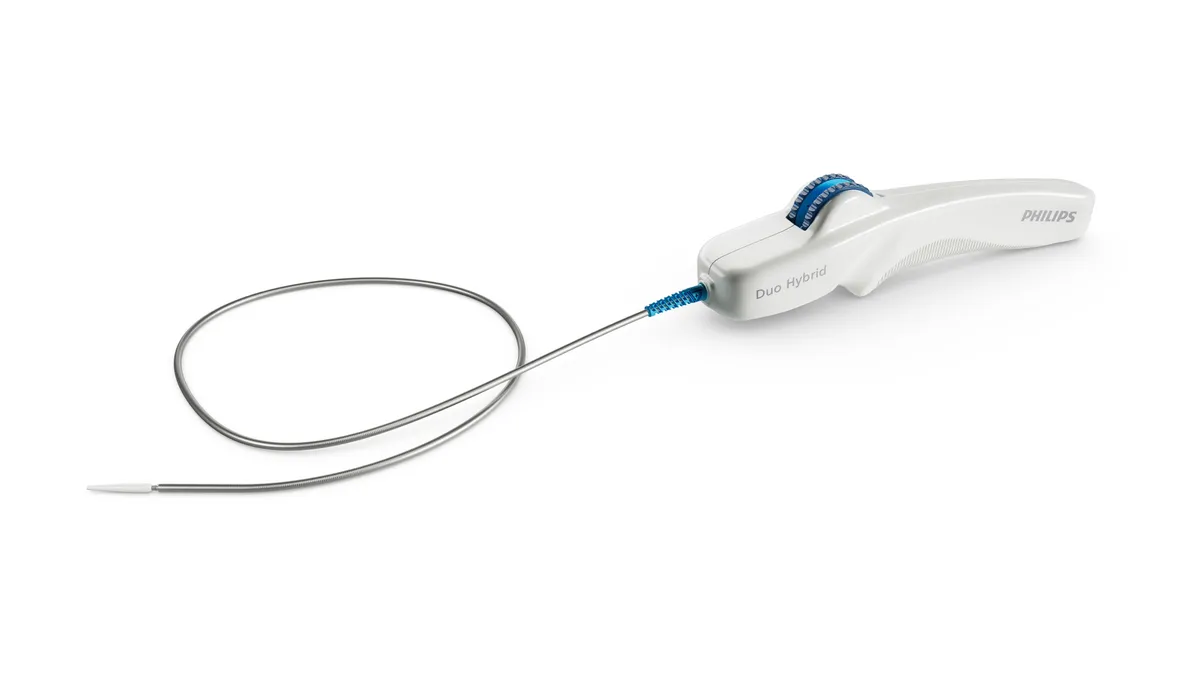
Philips launches stent system to restore blood flow in blocked veins
Philips acquired the device as part of its 227 million euro purchase of Vesper Medical in 2022.
By Nick Paul Taylor • June 13, 2024 -
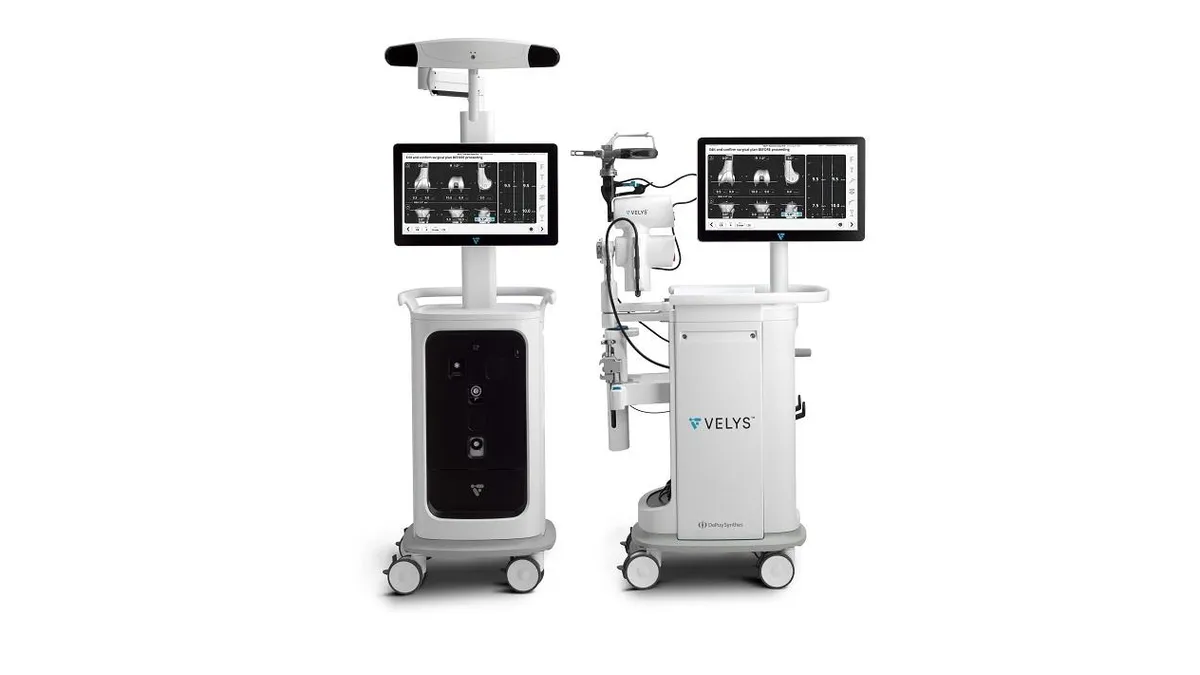
J&J wins expanded FDA clearance for knee surgery robot
Johnson & Johnson has identified Velys as a product that can help it regain market share from rivals such as Stryker.
By Nick Paul Taylor • June 10, 2024 -
Abbott wins CE mark for dual-chamber leadless pacemaker
Abbott sees the device as a product that can help its rhythm management business grow 6% to 7%.
By Nick Paul Taylor • June 7, 2024 -
Q&A
Stronger oversight of AI needed in medical devices, ECRI CEO says
Marcus Schabacker called for more upfront regulations and postmarket monitoring to better understand how AI features affect patient care.
By Elise Reuter • June 5, 2024 -
Lab industry sues to stop FDA’s new LDT rule
The American Clinical Laboratory Association argues the agency does not have the authority to enforce stricter standards on lab developed tests.
By Susan Kelly • May 30, 2024 -
New products take center stage in medtech’s latest earnings season
From pulsed field ablation devices to Intuitive's latest da Vinci robot, product launches dominated recent earnings calls.
By Ricky Zipp • May 28, 2024 -
Medline, Chinese manufacturer launch recalls for plastic syringes
Medline and Jiangsu Shenli Medical Production are at the center of the FDA’s probe of plastic syringes made in China.
By Ricky Zipp • May 24, 2024 -
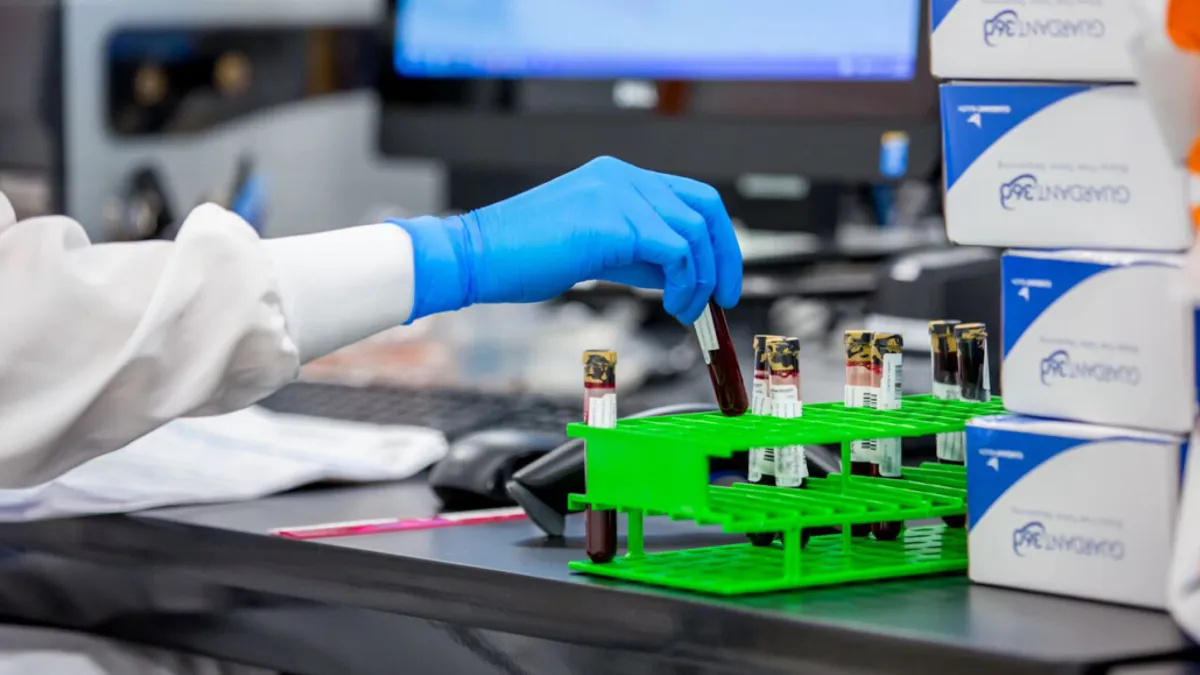
FDA panel backs Guardant’s blood test for colon cancer
“The favorable panel vote does not by any means imply this test will become an overnight success,” wrote William Blair analyst Andrew Brackmann.
By Susan Kelly • May 24, 2024 -
UK regulator proposes recognizing overseas medical device approvals
Companies with authorization in certain regions, including the EU and U.S., would be able to use their approvals to access Great Britain’s market.
By Nick Paul Taylor • May 24, 2024 -

 Retrieved from Hologic on May 23, 2024
Retrieved from Hologic on May 23, 2024
Hologic recalls more than 53,000 radiographic markers linked to 71 injuries
The Biozorb devices are staying on the market, but Hologic has asked people to report adverse events such as pain.
By Nick Paul Taylor • May 23, 2024 -
Medicare adviser sets recommendations for diabetes device evidence
A MEDCAC panel found time in range was an “extremely important” metric, but members were divided on whether quality of life measures should influence coverage.
By Elise Reuter • May 22, 2024 -
Guardant colon cancer test struggles to detect non-cancerous tumors, FDA says
Agency scientists highlighted the risk in materials posted ahead of a Thursday advisory panel meeting for the Guardant Health blood test.
By Susan Kelly • May 22, 2024 -
EMA updates advice on drug-device combinations under MDR, IVDR
The European Medicines Agency answered six new questions to clarify topics such as how companies can get advice on borderline products.
By Nick Paul Taylor • May 22, 2024 -
UK backs use of cancer treatment offered by Boston Scientific
A Boston Scientific executive said the guidance will expand access to a minimally invasive treatment for tumors that have metastasized to the liver.
By Nick Paul Taylor • May 20, 2024 -
FDA issues import alerts against 2 more plastic syringe manufacturers
Officials moved to stop the plastic syringes made by two Chinese manufacturers from entering the U.S. after finding quality system failings.
By Nick Paul Taylor • May 17, 2024 -
BD wins FDA approval for cervical cancer screening self-collection kit
Patients can self-collect the samples in locations such as retail pharmacies, which could encourage more people to get tested.
By Nick Paul Taylor • May 17, 2024 -
Getinge to limit US sales of heart devices after FDA safety warning
CEO Mattias Perjos said the action will have “some negative financial impact,” but the total effect will depend on customers' response.
By Nick Paul Taylor • May 16, 2024