FDA: Page 75
-
Medline, Vantage ethylene oxide emissions caused cancer, lawsuits claim
Commercial sterilizer Sterigenics, which was forced to shut down a Willowbrook medical device sterilizing plant in February, is defending itself against at least 32 lawsuits in the same state court over similar claims.
By David Lim • Aug. 29, 2019 -
AtriCure gets expanded FDA label for atrial exclusion device
AtriCure said it has strong growth from the AtriClip FLEX.V device and is confident growth rates for appendage management products will continue.
By Nick Paul Taylor • Aug. 28, 2019 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
FDA approves pediatric spine device via humanitarian pathway
The device is a less invasive alternative to spinal fusion for adolescents with idiopathic scoliosis.
By Nick Paul Taylor • Aug. 28, 2019 -
Titan Medical delays 510(k) submission until 2020 amid cash burn concerns
The company plans to use the delay to spread out its spending while working to implement the robotic surgery system's sterile instrument interface components, software improvements and training tools.
By David Lim • Aug. 27, 2019 -
Deep Dive
Pharmacogenetic test makers cheer UnitedHealth coverage. Other payers aren't there yet.
"I've been concerned that the hype around pharmacogenetics, at least for depression, has gotten out ahead of the data," James Potash, director of psychiatry at the Johns Hopkins University, said.
By Graison Dangor • Aug. 27, 2019 -
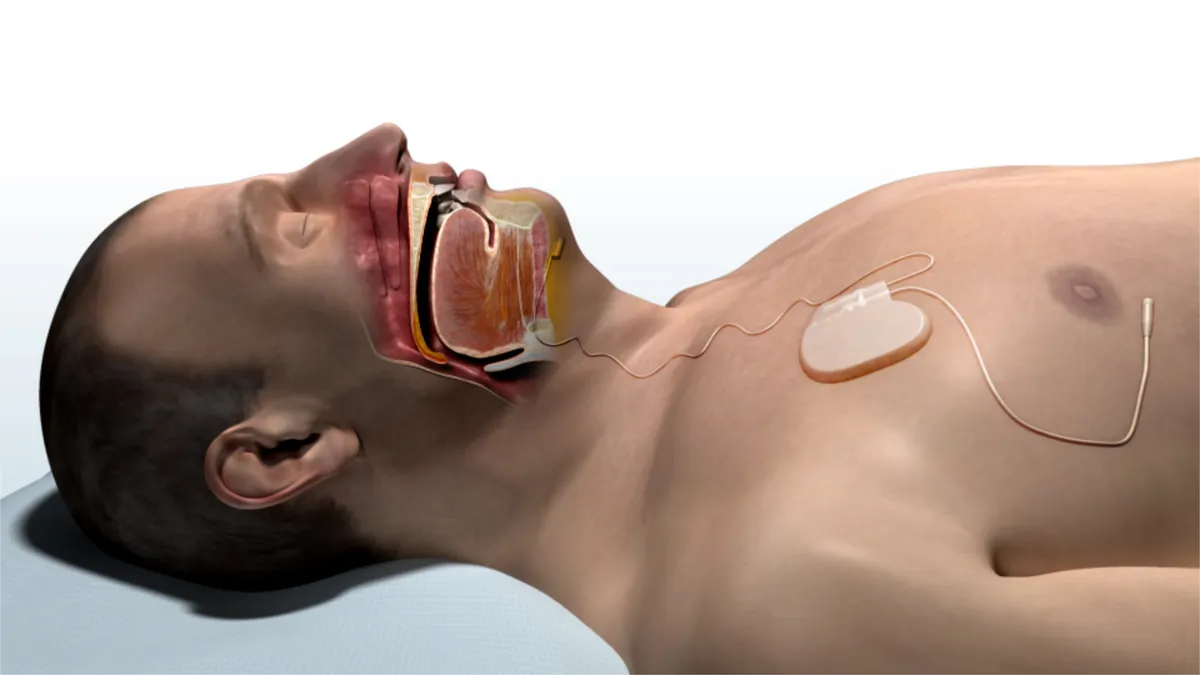
Medicare contractors back use of Inspire's sleep apnea device
Inspire Medical has now received draft decisions from four different Medicare contractors for the sleep apnea therapy, which could eliminate reimbursement "fatigue factors" the company says are limiting use of the product.
By Nick Paul Taylor • Updated Sept. 3, 2019 -
Lawmakers to update VALID this fall, but industry still clashing over details
With Republican Sen. Richard Burr as a new lead sponsor, Congress is working toward releasing an updated Leading-edge IVCT Development Act in the coming months, multiple sources familiar with the issue told MedTech Dive.
By David Lim • Aug. 26, 2019 -
Big data, cybersecurity among top FDA device center priorities
Reprocessing of devices and biocompatibility are also addressed in a report intended to incorporate new tech into regulatory decisions.
By David Lim • Aug. 23, 2019 -
Medicare draft decisions may boost Natera, Myriad tests
Cowen analysts called proposed local coverage for Natera's colorectal cancer test as "materially broader and quicker than expected," while documents on pharmacogenomic tests gave a mixed outlook for Myriad Genetics' products.
By Nick Paul Taylor • Aug. 23, 2019 -
Industry sounds alarm on EU single-use device reprocessing regs
There remains a risk that the European Commission will miss its November target and fail to finalize the draft policy before MDR comes into force.
By Nick Paul Taylor • Updated Aug. 27, 2019 -
ONC in talks with Congress, White House on third-party health app privacy
National Coordinator for Health Information Technology Don Rucker said there is bipartisan interest in taking action.
By Rebecca Pifer • Aug. 21, 2019 -
AdvaMed warns White House sterilization regs pose device shortage risk
The trade association's chief lobbyist told administration officials in a meeting an effective ban on ethylene oxide as a sterilant "poses an imminent public health threat through shortages of medical devices."
By David Lim • Aug. 21, 2019 -
FDA issues device sterilization warning letter
The notice accuses Innovative Sterilization Technologies of promoting the sterilization product in applications outside of the scope of its 510(k) clearance.
By Nick Paul Taylor • Aug. 21, 2019 -
FDA approves MRI labeling for Boston Scientific DBS system
CEO Michael Mahoney touted the growth of the company's deep brain stimulation products to investors during its second quarter earnings call.
By David Lim • Aug. 20, 2019 -
Italy's IMQ fourth notified body designated in EU
The firm joins Germany's Dekra and TÜV SÜD, as well as the United Kingdom's BSI, as the only four bodies designated less than a year before the new regulations take effect in May 2020.
By David Lim • Aug. 20, 2019 -
Edwards, Medtronic win expanded indications for low-risk TAVR patients
Roughly 165,000 low-risk patients per year in the U.S., Western Europe and Japan could now be eligible for the procedure, Medtronic estimates, a population both medtechs will be keen to treat.
By David Lim , Maria Rachal • Updated Aug. 16, 2019 -
UK plans for express medical products shipping service ahead of Brexit
The announcement of the shipping service comes as the government, led by new prime minister Boris Johnson, steps up no-deal scenario preparations and downplays risks.
By Nick Paul Taylor • Aug. 16, 2019 -
Hearing industry calls Bose self-fit hearing aid study flawed in complaint to FDA
The Hearing Industries Association letter argues the device's Phase II clinical study "does not provide enough evidence of effectiveness of the self-fitting method given the study's initial reliance on professionals for fitting."
By David Lim • Aug. 15, 2019 -
Abbott poised to profit from CMS review of TMVR coverage
Analysts at Cowen estimate CMS coverage in a newly considered indication could triple the number of patients eligible for transcatheter mitral valve repair.
By Nick Paul Taylor • Aug. 15, 2019 -
Industry backs Australia's device identifier plan with caveats
The responses showed broad support for a unique device identification system based on global guidelines, but the Therapeutic Goods Administration also faced some pushback from J&J and Roche.
By Nick Paul Taylor • Aug. 15, 2019 -
Australia weighs in on paclitaxel-coated balloons
The Therapeutic Goods Administration said data on adverse events in Australia haven't shown a signal like the one described by FDA.
By Susan Kelly • Aug. 13, 2019 -
USTR: 10% tariffs on 'certain' electronics from China delayed to Dec. 15
Chinese imports not included on USTR's forthcoming list will still face 10% duties on Sept. 1, as President Donald Trump announced earlier this month.
By Shefali Kapadia • Updated Aug. 13, 2019 -
Concept Medical nabs breakthrough designation for sirolimus-coated balloon for PAD
If granted FDA marketing authorization, the device may compete with paclitaxel devices sold by BD, Medtronic, Boston Scientific, Philips and Cook Medical.
By David Lim • Aug. 13, 2019 -
China grants innovative device designation to Novocure's Optune
Novocure's Chinese partner now gets the opportunity for more contact with regulators and an expedited review in a market estimated at $96.3 billion this year, up from $53.6 billion in 2016.
By Nick Paul Taylor • Aug. 13, 2019 -
CMS rejects industry bid to boost payments for device-based opioid alternatives
The agency said evidence submitted was plagued by potential conflicts of interest, inadequate sample sizes and lack of peer review.
By David Lim • Aug. 12, 2019