FDA: Page 70
-
Hahn confirmed as next FDA commissioner in 72-18 Senate vote
Stephen Hahn's swift confirmation won applause from lawmakers, former FDA commissioners and industry groups.
By David Lim • Dec. 12, 2019 -
EC medical device group sheds new light on MDR, IVDR sampling codes
The Medical Device Coordination Group also issued a guidance explaining codes used to define the scope of a notified body's designation.
By Nick Paul Taylor • Dec. 12, 2019 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Grassley, Warren press FDA to issue over-the-counter hearing aid regulations
The agency missed its estimate that the rules would come out last month, but a spokesperson said that timeline was "not intended to be a precise estimate."
By David Lim • Dec. 9, 2019 -
House bill would speed CMS coverage for breakthrough medtech
The bipartisan legislation, reintroduced on Friday, promises faster access to new medical technologies by requiring Medicare to cover devices approved through FDA's breakthrough pathway during a three-year transitional period.
By Susan Kelly • Dec. 9, 2019 -
FDA proposes alternative 510(k) criteria for MRI coils
The draft guidance would permit manufacturers to use a set of performance criteria to demonstrate substantial equivalence, instead of a direct comparison to a predicate device.
By Susan Kelly • Dec. 9, 2019 -
Impeachment clouds year-end medical device tax repeal, industry priorities
AdvaMed argues the primary obstacle to repealing the tax is not the revenue the government would no longer collect, but a crowded year-end legislative agenda and impeachment proceedings against President Donald Trump.
By David Lim • Dec. 9, 2019 -
Dive Awards
Disruption of the Year: EU MDR notified bodies shortage
The new European Union Medical Device Regulation requires more devices to undergo notified body review than ever before. But there are worries there aren't enough notified bodies to handle the workload.
By Dana Elfin • Dec. 9, 2019 -
Dive Awards
Regulatory Development of the Year: 510(k) changes inch along
New efforts by FDA to build out the program come years after the Institute of Medicine recommended scrapping it. More than 80% of devices are cleared through the pathway, so small changes can be meaningful to product safety.
By David Lim • Dec. 9, 2019 -
Dive Awards
The MedTech Dive Awards of 2019
From TAVR's takeoff to FDA's new approaches to premarket review, these are the industry forces that left their mark on medical technology this year.
Dec. 9, 2019 -
EPA wants more data from ethylene oxide sterilizers, strikes device-friendly tone
Administrator Andrew Wheeler said "medical device sterilization is vital to protecting public health" on release of a notice ahead of rulemaking that could impact sterilizers reliant on the carcinogenic gas.
By David Lim • Dec. 6, 2019 -
FDA grades new Medtronic SynchroMed II recall as Class I event
The voluntary move is the latest in a series of safety actions involving the implantable drug infusion pump, which was the focus of a 2015 consent decree between the company and the government.
By Nick Paul Taylor • Updated Dec. 10, 2019 -
Vicarious' surgical robot gets FDA breakthrough status
The company also announced Scott Huennekens, former CEO of Google and Johnson & Johnson's robotic surgery joint venture, joined its board of directors.
By Nick Paul Taylor • Dec. 5, 2019 -
Sens. Wyden, Booker push CMS, FTC, insurers on bias in algorithms
The lawmakers want an investigation into how use of the algorithms may lead to discrimination against marginalized populations.
By Susan Kelly • Dec. 4, 2019 -
EU Parliament panel adopts MDR delay for some Class I devices, full nod likely
One committee member called out the unusual nature of making late-in-the-game substantive regulatory changes through the corrigendum, a procedure typically meant for correcting technicalities and inconsistencies.
By Dana Elfin • Dec. 4, 2019 -
FDA commissioner nominee backs 'proactive, not passive' approach to device safety
While Stephen Hahn shed more light on his thoughts on certain medical device topics, he declined to say FDA scientific judgments of medical devices should be held to the same regulatory standards as drugs in response to a question.
By David Lim • Dec. 4, 2019 -
Hospitals sue HHS, warning price transparency rule would chill competition, crash computers
An agency spokeswoman shot back that hospitals "should be ashamed that they aren't willing to provide American patients the cost of a service before they purchase it."
By Samantha Liss • Dec. 4, 2019 -
Contract sterilizer of devices draws FDA warning over validation issues
American Contract Systems received multiple complaints about the presence of foreign matter in sealed sterilization bags, the agency said.
By Nick Paul Taylor • Dec. 4, 2019 -
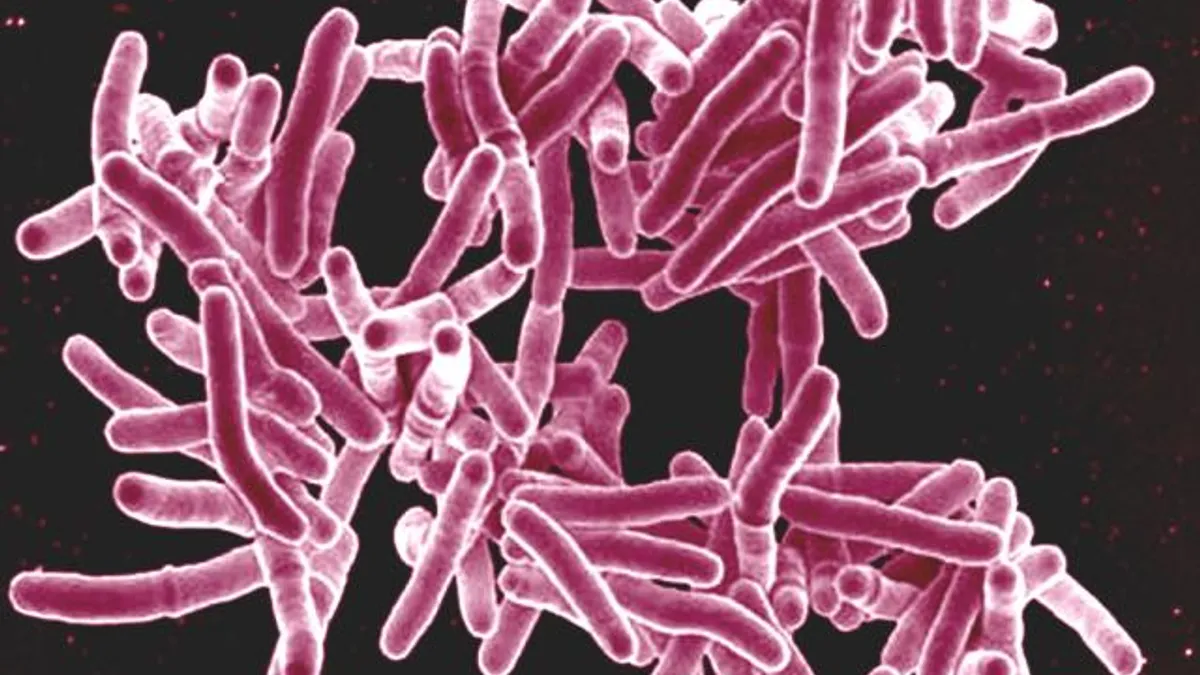
FDA approves TB test from Qiagen and DiaSorin
The approval could be a win-win for both companies, bringing Qiagen's TB blood test to DiaSorin's Liaison immunoassay analyzers while expanding the testing menu for Liaison.
By Nick Paul Taylor • Dec. 2, 2019 -
With 6 months until new EU medical device rules, what's the state of play?
Preparing for the May 26, 2020, implementation of the EU's Medical Device Regulation hasn't been easy, given the lack of notified bodies designated to review devices under the more stringent standards.
By Dana Elfin • Updated Nov. 26, 2019 -
Mandatory CMS radiation oncology model goes on the backburner
Originally, the agency was eying an implementation date as early as Jan. 1, but the new regulatory agenda lists July 2022 as a target date for the bundled payment model.
By David Lim • Nov. 26, 2019 -
FDA launches pilot to speed new ethylene oxide sterilization methods
Device makers should also reduce paper in packaging "as soon as possible" to trim the amount of the gas required for effective sterilization. The agency also named its innovation challenge winners.
By David Lim • Nov. 25, 2019 -

 Retrieved from U.S. Environmental Protection Agency.
Retrieved from U.S. Environmental Protection Agency.
AdvaMed lobbied EPA chief Wheeler on cancer report amid delay in ethylene oxide regs
A bipartisan congressional task force is pushing EPA to issue ethylene oxide regulations that rely on EPA's 2016 report on the toxicity of the gas.
By David Lim • Nov. 22, 2019 -
Medtronic's recovering DCB segment scores FDA approval of IN.PACT AV
The approval allows Medtronic to sell the paclitaxel-coated balloon as a treatment for failing arteriovenous access in dialysis patients.
By Nick Paul Taylor • Nov. 22, 2019 -
Rare medical device ban, OTC hearing aids make FDA year-end to-do list
Medical device manufacturers concerned about a federal crackdown on commercial ethylene oxide sterilizers can breathe a sigh of relief as the EPA says a timeline for final regulations remains to be determined.
By David Lim • Nov. 21, 2019 -
Transcatheter device to treat heart failure gets breakthrough designation
BioVentrix is enrolling 120 subjects in a clinical trial meant to support U.S. marketing authorization for its minimally invasive alternative to conventional surgical ventricular reconstruction.
By Nick Paul Taylor • Nov. 21, 2019