FDA: Page 7
-
FDA warns 2 Chinese labs for oversight failures, animal care violations
The FDA said the problems “raise concerns about the quality and integrity of data generated by the labs,” which provide third-party testing and validation data services for device firms.
By Nick Paul Taylor • Sept. 12, 2024 -
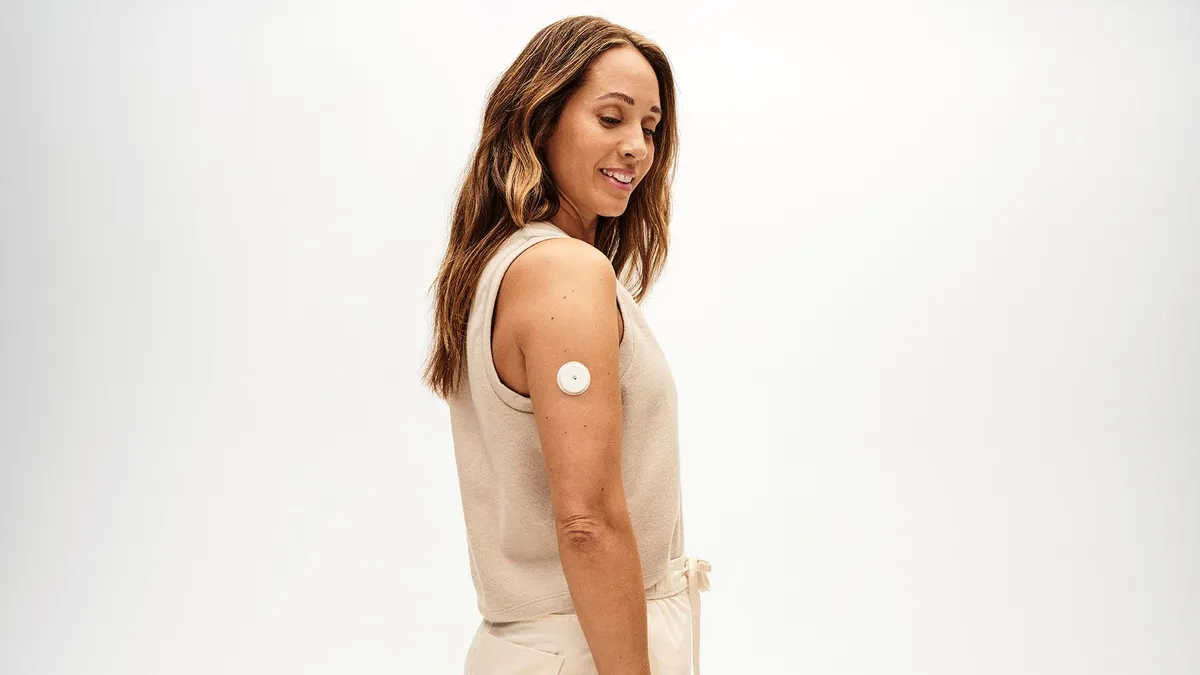
Dexcom, Abbott OTC glucose sensors add to busy year for diabetes tech
New over-the-counter sensors, an Abbott-Medtronic partnership and Roche’s first CGM are among diabetes technology’s top stories so far in 2024. Check out MedTech Dive’s roundup of the latest news.
By Ricky Zipp • Sept. 11, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
FDA to investigate presence of metals in tampons
Members of the Democratic Women’s Caucus last week urged FDA Commissioner Robert Califf to address the safety concerns.
By Nick Paul Taylor • Sept. 11, 2024 -
Lab group urges lawmakers to rescind FDA final rule on LDTs
A survey found 48% of labs will discontinue LDTs if they do not qualify for an exception under the FDA final rule.
By Nick Paul Taylor • Sept. 9, 2024 -

 Retrieved from The Food and Drug Administration.
Retrieved from The Food and Drug Administration.
FDA cracks down on ozone cleaners for CPAP machines
The agency sent warning letters to four companies and reminded the public it has not authorized any devices for cleaning or disinfecting CPAPs.
By Elise Reuter • Sept. 5, 2024 -
European heart group recommends renal denervation for some patients
The European Society of Cardiology said the treatment may be considered for certain patients with uncontrolled, drug-resistant high blood pressure but outlined lingering concerns.
By Nick Paul Taylor • Sept. 4, 2024 -
Illumina avoids fine for Grail purchase in European court victory
Illumina will avoid a penalty of 432 million euros after a court ruled the European Commission did not have jurisdiction to challenge the company’s Grail acquisition.
By Susan Kelly • Sept. 3, 2024 -
ARPA-H program to focus on AI degradation in medical tools
The agency will fund work to identify and auto-correct AI-enabled tools that are misaligned with their underlying training data.
By Nick Paul Taylor • Sept. 3, 2024 -
FDA finalizes Voluntary Malfunction Summary Reporting guidance
The agency clarified when companies can submit device malfunction reports in a quarterly summary, instead of individual reports every 30 days.
By Nick Paul Taylor • Aug. 30, 2024 -
Boston Scientific receives CE mark for updated TAVR technology
Changes include the addition of a larger valve size that company executives have said will open up 25% of the market in Europe.
By Nick Paul Taylor • Aug. 28, 2024 -
Illumina wins FDA approval for companion diagnostic cancer test
Physicians can use the test to identify people eligible for treatment with Bayer’s Vitrakvi and Eli Lilly’s Retevmo, which target cancers that have specific genetic features.
By Nick Paul Taylor • Aug. 28, 2024 -
FDA seeks feedback on predetermined change control plans
The guidance describes changes companies can make to medical devices without filing a new 510(k) or premarket approval supplement.
By Nick Paul Taylor • Aug. 26, 2024 -
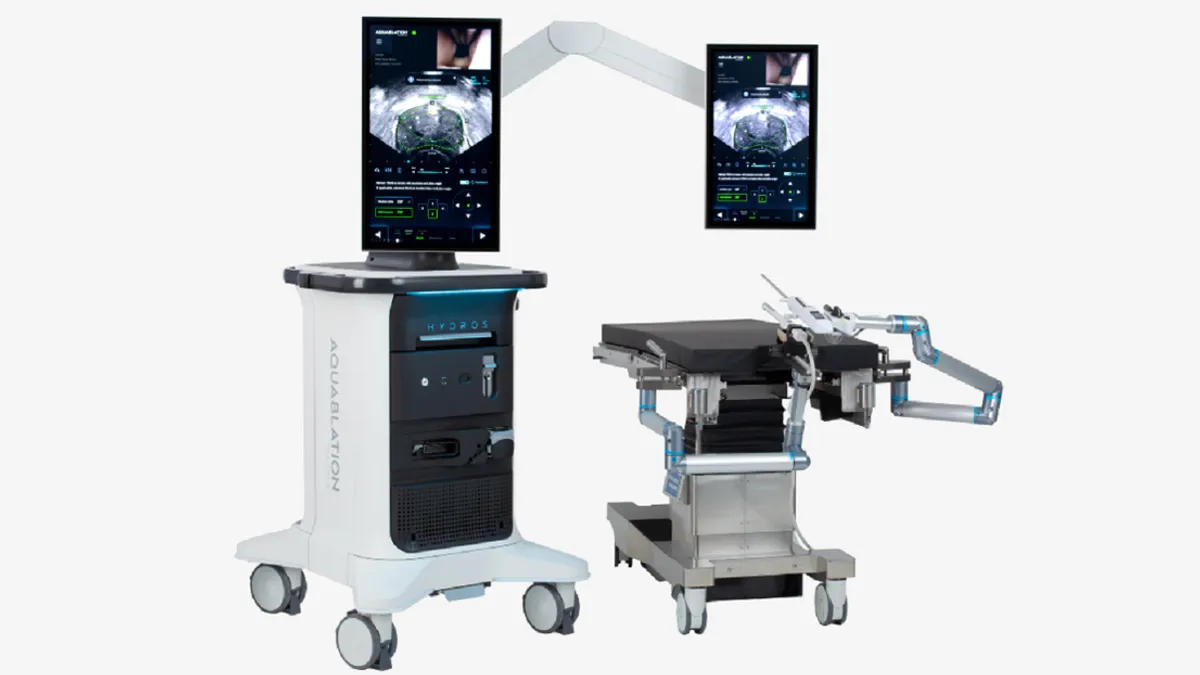
Procept wins FDA clearance for updated prostate surgery robot
Procept Biorobotics has added AI capabilities and switched to a single-use scope to support mass-market adoption.
By Nick Paul Taylor • Aug. 22, 2024 -
FDA defends Shuren’s tenure after report raises ethics concerns
The FDA backed Jeff Shuren, the agency’s former device leader, amid questions raised by The New York Times. Still, a spokesperson said the FDA has advised Shuren to take greater caution in managing recusal obligations.
By Susan Kelly • Aug. 22, 2024 -

 "Government Accountability Office Building" by kafka4prez is licensed under CC BY-SA 2.0
"Government Accountability Office Building" by kafka4prez is licensed under CC BY-SA 2.0
FDA’s plan to expand device surveillance faces challenges, GAO finds
Funding issues and limited use of unique device identifiers are hurdles to expanding an FDA system to monitor medical device safety, the federal watchdog said.
By Nick Paul Taylor • Aug. 21, 2024 -
FDA OKs first at-home syphilis test
NowDiagnostics’ over-the-counter test can return results in 15 minutes with a single drop of blood.
By Ricky Zipp • Aug. 19, 2024 -
Boston Scientific resubmits Silk Road merger filing, giving FTC more review time
A Boston Scientific spokesperson said the company still expects to close its $1.26 billion purchase of Silk Road Medical in the second half of the year.
By Ricky Zipp • Aug. 16, 2024 -

 Erin Scott / U.S. White House. Retrieved from Flickr.
Erin Scott / U.S. White House. Retrieved from Flickr.
Biden pledges $150M for medical tech to improve cancer surgery
Awards include funding for microscopy and imaging tools to help surgeons ensure all cancer is removed during a procedure.
By Elise Reuter • Aug. 16, 2024 -
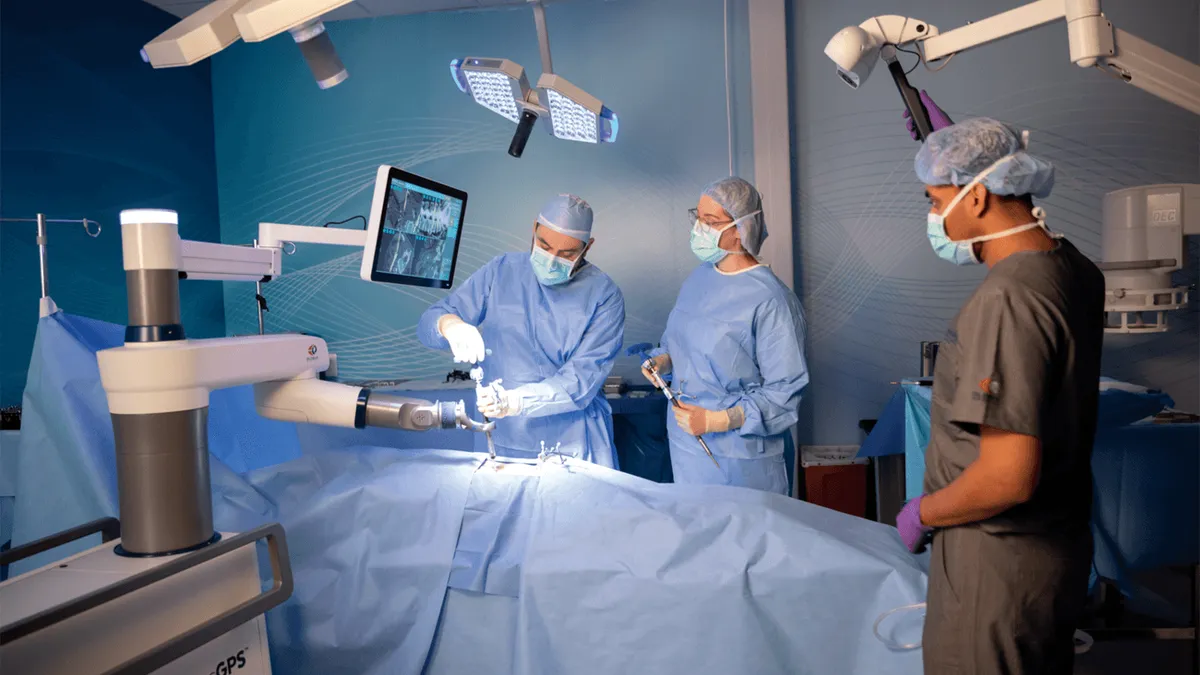
 Retrieved from Globus Medical on August 14, 2024
Retrieved from Globus Medical on August 14, 2024
Globus receives warning letter over spine navigation robot
The FDA said Globus delayed reporting complaints about misplaced screws, despite information that “reasonably suggests” the Excelsius GPS system malfunctioned.
By Nick Paul Taylor • Aug. 14, 2024 -
Medtronic wins FDA approval for asleep deep brain stimulation
Medtronic said Monday it is the first company to receive FDA approval to offer DBS surgery while a patient is asleep or awake.
By Nick Paul Taylor • Aug. 13, 2024 -
CMS finalizes notice on Medicare coverage for breakthrough devices
Through the new pathway, called Transitional Coverage for Emerging Technologies, the CMS will consider five medical device candidates yearly for national coverage.
By Elise Reuter • Aug. 8, 2024 -
FDA seeks feedback on health equity for medical devices
The agency plans to develop a framework for when devices should be evaluated in diverse populations to support marketing authorization.
By Nick Paul Taylor • Aug. 6, 2024 -
Labcorp wins FDA OK for pan-solid tumor liquid biopsy
Shakti Ramkissoon, Labcorp’s medical lead for oncology, said the test could allow laboratories and oncologists to profile solid tumors when tissue samples are limited or unavailable.
By Nick Paul Taylor • Aug. 6, 2024 -
Inspire wins FDA approval for obstructive sleep apnea neurostimulator therapy
Inspire plans to start a soft launch of the device late in 2024 and move into a full release in 2025.
By Nick Paul Taylor • Aug. 5, 2024 -
Experts fear patient harm from FDA’s lab developed test rule
Decreased access to diagnostic tests, worsening patient care and practice closures are among the concerns the AMA and others have raised as the regulation takes effect.
By Susan Kelly • Aug. 5, 2024