FDA: Page 64
-
House Dems carve out $75B for coronavirus testing, contact tracing in bid for new relief package
The $3 trillion bill passed the House by a vote of 208-199 on Friday. It's Democrats' opening gambit for the next wave of congressional action, but Republicans say they're not in a hurry to approve new funding.
By Susan Kelly • Updated May 18, 2020 -
FDA flags 2nd high-risk recall of embolectomy catheters in recent weeks
The potential for a detached catheter tip to damage blood vessels and cause serious injuries or death underpinned the agency’s Class I label for an Applied Medical recall on Tuesday, seven months after the company initiated the recall.
By Nick Paul Taylor • May 13, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Eko algorithm gets FDA nod for cardiac screening of COVID-19 patients
Developed with the Mayo Clinic, Eko's algorithm gained breakthrough device status in December and review was further accelerated given its potential to identify patients at higher risk of poor outcomes with the coronavirus.
By Nick Paul Taylor • May 13, 2020 -
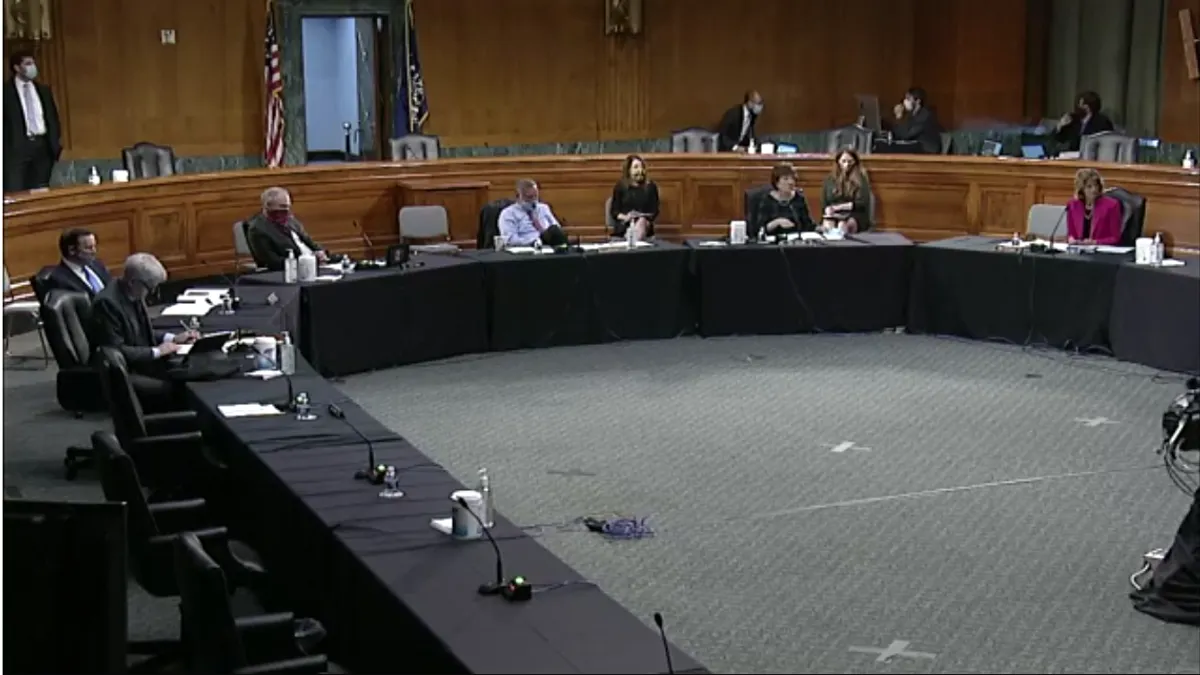
As Trump pushes states to reopen, Fauci warns against lifting COVID-19 restrictions too soon
The U.S. doesn't have the necessary testing and surveillance infrastructure in place to prep for a fall resurgence of the coronavirus, a second wave that's "entirely conceivable and possible," Fauci told a Senate panel.
By Rebecca Pifer • May 12, 2020 -
Abiomed faces familiar reimbursement fluctuation in CMS proposal
An almost 25% reduction to a key heart implant procedure payment code, plus backing for three breakthrough-designated devices, are among the policies outlined in a Medicare proposed rule released Monday afternoon.
By Maria Rachal • May 12, 2020 -
LabCorp at-home collection test kit for coronavirus goes mainstream, as states get $11B for testing efforts
The tests, originally only offered to frontline workers, are now available even to individuals without symptoms who may have been exposed to the virus. Still, clinical labs say more support is needed to further ramp up capacity.
By Greg Slabodkin • May 12, 2020 -
FDA, CDC drawing up plan to restart routine facility inspections
A phased approach is in the works to reintroduce certain oversight that's been on hold both domestically and internationally since the coronavirus outbreak reached pandemic level in March.
By Nick Paul Taylor • May 12, 2020 -
Latest Abbott coronavirus antibody test receives FDA emergency use OK
The EUA is the company's second for a test detecting the IgG antibody, using a different machine than the first. Across both, it plans to ship almost 30 million units worldwide in May and double that amount in June.
By Greg Slabodkin • May 11, 2020 -
1st antigen test to detect coronavirus gets FDA nod
The agency, which gave the green light on Saturday to Quidel, expects the tests to be cheaper to make than PCR tests.
By Nick Paul Taylor • May 11, 2020 -
FDA details COVID-19 era reporting rules to stymie medical device shortages
Under new powers granted by the CARES Act for the duration of the health crisis, the guidance to industry lays out when and how to notify the agency about changes that could affect product availability.
By Nick Paul Taylor • May 7, 2020 -
Notified bodies saw CE marks spike in 2019 ahead of now-delayed MDR
Team-NB attributed the surge to an industry push to get certified under the outgoing Medical Device Directive, before the COVID-19 pandemic led the EU to postpone the new regulations start date by a year.
By Nick Paul Taylor • May 6, 2020 -
FDA beefs up coronavirus antibody test regs critics called 'recipe for disaster'
The Association of Public Health Laboratories praised the move. "This revised policy makes a lot of sense and should have been in place over the last six weeks," the group's CEO Scott Becker said.
By Greg Slabodkin • May 4, 2020 -
7 states team up to buy $5B in ventilators, PPE to meet COVID-19 demand
New York, New Jersey, Connecticut, Pennsylvania, Delaware, Rhode Island and Massachusetts are aiming to use their combined purchasing power to drive down prices amid rivalry with other states and even the federal government.
By Rebecca Pifer • May 4, 2020 -
CMS eases rules on COVID-19 diagnostics, antibody testing
A written practitioner’s order is no longer needed to obtain Medicare payment for diagnostic testing. The agency will also cover serology testing, including certain FDA-authorized tests for which patients collect samples at home.
By Hailey Mensik • May 1, 2020 -
Medtronic continuous dialysis system designed for small children gets FDA nod
The De Novo authorization, one of a only a handful doled out by the agency so far this year, gives Medtronic a chance to dominate a pediatric niche. Baxter tested a similar system but terminated the study in 2018.
By Nick Paul Taylor • May 1, 2020 -
AdvaMed pushes US to lift tariffs on Chinese imports of COVID-19 devices
The trade group wants indefinite exemptions for imaging components and devices used in ventilators, among other items. It also floated a compromise: re-imposing them one year after the pandemic passes.
By Nick Paul Taylor • April 30, 2020 -

 "State Public Health Laboratory in Exton Tests for COVID-19" by Governor Tom Wolf is licensed under CC BY 2.0
"State Public Health Laboratory in Exton Tests for COVID-19" by Governor Tom Wolf is licensed under CC BY 2.0
LabCorp, Quest expand COVID-19 antibody testing nationwide
Amid the moves by the giant commercial labs, a White House plan released Monday says the administration is still exploring how to improve the reliability of these products.
By Greg Slabodkin • April 28, 2020 -
FDA encourages remote review of digital pathology slides amid COVID-19
The agency's latest effort to support greater access to medical devices during the pandemic follows a move by CMS that opened the door for pathologists to work away from the lab.
By Susan Kelly • April 27, 2020 -

 Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
Gottlieb, Slavitt pitch $46.5B plan for COVID-19 contact tracing, isolation
Primary care doctors should be the main referral source for testing and contact tracing, according to a letter penned by a bipartisan group including former CMS head Andy Slavitt and former FDA Commissioner Scott Gottlieb.
By Shannon Muchmore • April 27, 2020 -
CMS hits pause on speeding Medicare payments, including for DME suppliers
The Trump administration is suspending accelerated Medicare pay to doctors and durable medical equipment suppliers, and is re-evaluating hospital requests, after another round of emergency funding was signed into law Friday.
By Rebecca Pifer • April 27, 2020 -
Abbott, DiaSorin coronavirus antibody tests get EUAs as lawmakers question others on market
As more diagnostics assessing possible immunity to the virus win FDA emergency use authorization, criticism is mounting over the accuracy of products the agency allowed on the market without the designation.
By Greg Slabodkin • April 27, 2020 -
Digital therapeutic makers see opportunity as pandemic prompts FDA to ease rules
Akili Interactive Labs is rolling out its video game treatment for ADHD for the first time, while Pear Therapeutics weighs how products still in its pipeline may apply during the coronavirus crisis.
By Maria Rachal • April 24, 2020 -
It's official: EU finalizes 1-year MDR delay
The European Commission listed a 13th notified body designated for Medical Device Regulation-related work in its official database Saturday, the first new firm announced in over a month and the fifth based in Germany.
By Maria Rachal • Updated April 27, 2020 -
FDA moves to boost access to imaging systems during pandemic
The agency is allowing devices to be modified for portable use to enable imaging outside of hospitals in an effort to potentially slow the spread of COVID-19.
By Nick Paul Taylor • April 24, 2020 -
FDA grants breakthrough status to heart failure, stent graft tech
The newly disclosed breakthrough device designations follow ones awarded to a speech therapy app and a treatment for stable vertebral compression fractures in recent weeks.
By Nick Paul Taylor • April 24, 2020