FDA: Page 60
-
FEMA pitches voluntary DPA pact to bolster medical supply chain during pandemic
The Defense Production Act allows private companies to enter agreements with the government while avoiding antitrust liability. AdvaMed wants to participate but FEMA has not yet extended an invite.
By Greg Slabodkin • Aug. 18, 2020 -
Thermo Fisher COVID-19 test flagged for false positive and negative results
An FDA alert on Monday follows a quarter in which coronavirus-related products added $1.3 billion to the medtech's sales, and after it scored an HHS contract to provide processing instruments to labs.
By Nick Paul Taylor • Aug. 18, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
FDA looks to downclass noninvasive bone growth stimulators, incumbents not on board
The agency made a case for moving the devices to less-rigorous Class II regulation in a proposal opposed by manufacturers with products already on the market such as Orthofix.
By Susan Kelly • Aug. 17, 2020 -
FDA OKs Yale COVID-19 saliva test removing RNA extraction step
While the agency called the method potentially groundbreaking, one public health expert cautioned that the assay must be performed in highly specialized labs and is not considered a rapid test.
By Nick Paul Taylor • Aug. 17, 2020 -

 "White House Press Briefing". Retrieved from The White House.
"White House Press Briefing". Retrieved from The White House.
HHS commits $6.5M to add Thermo Fisher, Beckman Coulter COVID-19 test supplies to Aegis, Sonic labs
The federal funding to scale testing, still dwarfed by government spending on vaccines, came the same week labs and providers urged the administration to update testing prioritization guidelines given limited resources.
By Greg Slabodkin • Aug. 14, 2020 -
Hospitals, docs pitch roadmap to keep essential surgeries going even with coronavirus surges
While many medtechs cast the worst of the pandemic's impact in the rearview mirror in recent financial reports, concerns from healthcare industry groups reflect a more nuanced picture.
By Nick Paul Taylor • Aug. 14, 2020 -
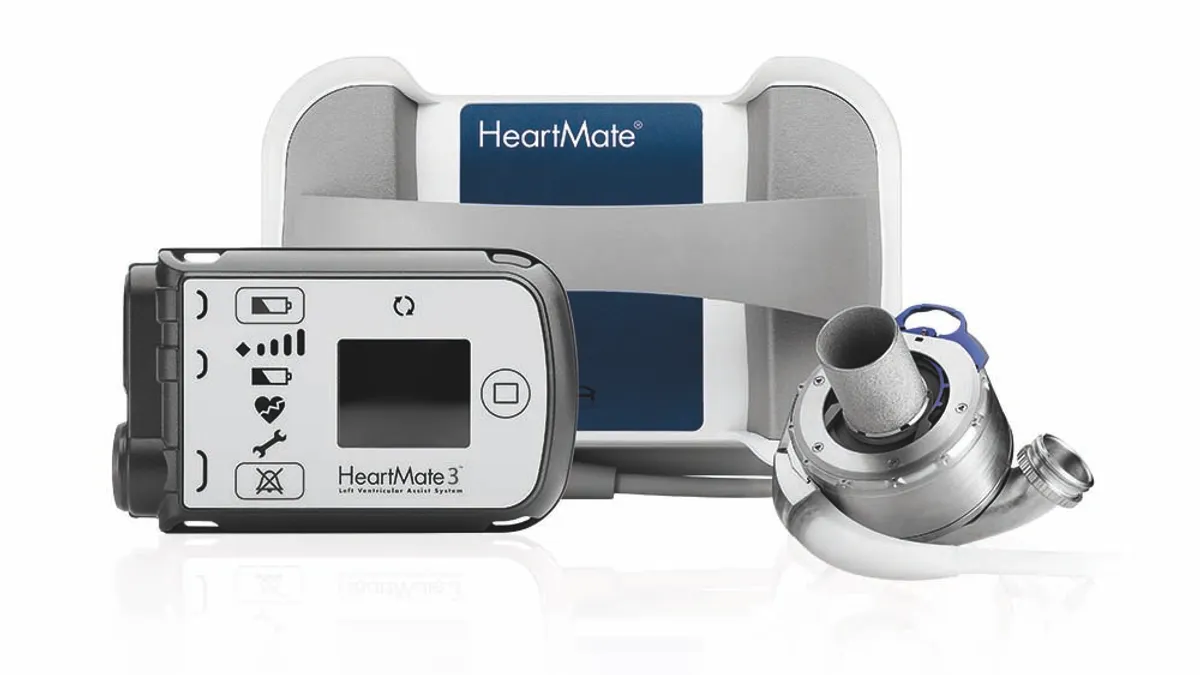
CMS seeks to ease rules on artificial heart, ventricular assist device coverage
Potential eligibility changes for advanced heart failure patients drew positive feedback from Abbott and Medtronic, while removal of a coverage with evidence development policy has been opposed by several cardiologist groups.
By Maria Rachal • Aug. 13, 2020 -
Watchdog again pushes CMS to add UDIs to claim forms
The HHS Office of Inspector General said that without a place to ask for device-specific information, it's difficult for the agency to track billions of dollars in costs related to failed devices.
By Nick Paul Taylor • Aug. 13, 2020 -
Coronavirus muddies FDA's trajectory on device warning letters
An agency official said going into 2020 that its devices center would be better equipped to use the compliance tool in a timely fashion. But a dry spell for on-site surveillance may undermine any noticeable rebound.
By Maria Rachal • Updated Aug. 12, 2020 -
FDA posts Essure adverse events pulled from social media
The spreadsheet includes details of 53 deaths and 1,376 reports of serious injuries related to the controversial birth control implant but the agency is unsure if it contains duplicate reports.
By Nick Paul Taylor • Updated Aug. 14, 2020 -
BD's FDA filing to update recalled Alaris pumps delayed by 6 months
The medtech planned to rectify the situation with FDA this year, but that schedule has slipped as it continues system testing and ongoing discussions with the agency. BD estimates remediation costs will total $200 million.
By Greg Slabodkin • Aug. 10, 2020 -
Notified bodies list for incoming EU regs hits 20 with designation of DQS for MDR
The 16 notified bodies now allowed to complete work under the Medical Device Regulation and the four OK'd under its In Vitro Diagnostic counterpart together meet the goal that authorities had hoped to achieve by the start of 2020.
By Nick Paul Taylor • Aug. 10, 2020 -
10 states now on board to buy Quidel, BD antigen tests
With the latest additions of Arkansas and Rhode Island, there are now five Democratic and five Republican governors each planning to acquire half a million of the rapid point-of-care diagnostics from the medtechs.
By Greg Slabodkin • Updated Aug. 19, 2020 -
Medtronic, in catch-up to Axonics, wins nod for rechargeable bladder and bowel control device
After leading the sacral neuromodulation market for two decades, Medtronic is defending its turf with FDA approval of a product it contends is 80% smaller than its current device and 50% smaller than Axonics' rechargeable system.
By Susan Kelly • Aug. 4, 2020 -
CMS proposes eliminating inpatient-only list, in potential boon for ASCs
The agency would begin by removing about 300 musculoskeletal-related services, which could further accelerate ambulatory surgery centers' important role as medtech customers.
By Shannon Muchmore • Aug. 4, 2020 -
CMS pitches key telehealth changes following Trump executive order
In its 2021 physician fee schedule, the agency is proposing making permanent almost two dozen new telehealth codes and clarified certain provisions around remote patient monitoring.
By Rebecca Pifer • Aug. 4, 2020 -
J&J gets FDA breakthrough status for robot-assisted ablation device
The designation gives the product, which combines technologies Johnson & Johnson acquired via takeovers of NeuWave Medical and Auris Health, more regulatory support and potentially speedier review.
By Nick Paul Taylor • July 31, 2020 -
Falling FDA personnel costs soften 2021 medical device user fee hike
The agency's required user fees for medical device submissions will rise 7.2% beginning in October.
By Nick Paul Taylor • July 30, 2020 -
FDA finalizes guidance on multiple-function devices after industry feedback
The agency clarified its policies and regulatory practices governing devices that have at least one distinct function and one other purpose that is not subject to premarket review.
By Susan Kelly • July 29, 2020 -
Color targets supply, staffing bottlenecks with updated coronavirus test EUA
The population health technology company's test uses dry nasal swabs, eliminating the need for the viral transport media that's been in short supply and limited scaling by lab giants Quest and LabCorp.
By Nick Paul Taylor • July 28, 2020 -
Deep Dive
Coronavirus testing hits a wall: Where do we go from here?
As LabCorp and Quest are backlogged in delivering molecular test results, some public health officials are calling for a shift in testing approach from slow and accurate to fast and good enough to meet demand.
By Greg Slabodkin • July 27, 2020 -
Medtech and COVID-19: 6 months into the public health emergency
MedTech Dive examines where the industry stands with testing and the future of those emergency use-authorized diagnostics, as well as how it's adapting to a rebound in elective surgeries and various supply chain challenges.
July 27, 2020 -
Diagnostics makers must plan for EUA products to have post-pandemic future
An extension to the public health emergency formalized a reprieve for emergency use authorizations, but manufacturers should act now if they want products to stay on the market when the crisis ends, compliance experts say.
By Greg Slabodkin • July 27, 2020 -
LabCorp COVID-19 test gets 1st FDA OK for asymptomatic screening
U.S. regulators simultaneously allowed the LabCorp test to be used in sample pooling, following the first such nod to a Quest Diagnostics test a week ago.
By Nick Paul Taylor • July 27, 2020 -
HHS renews COVID-19 public health emergency
The extension supported by AdvaMed, the American Clinical Laboratory Association and American Hospital Association takes effect Saturday and opens the door for FDA to keep issuing emergency use authorizations.
By Shannon Muchmore • July 23, 2020