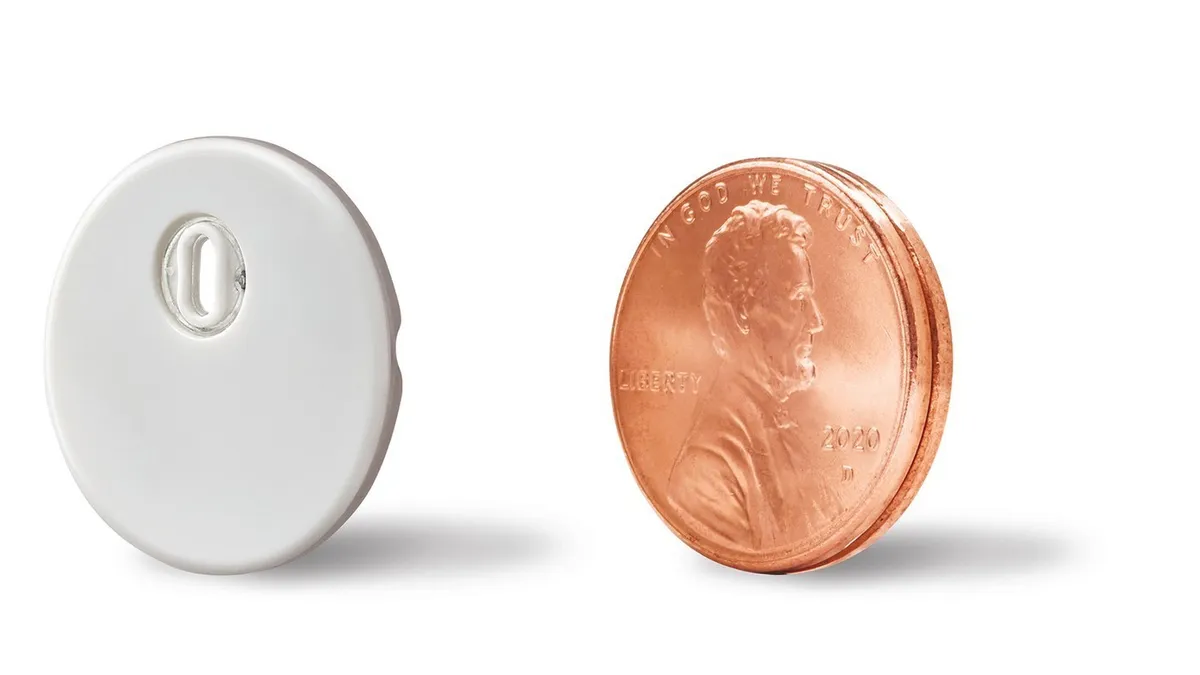FDA: Page 58
-
Abbott gets CE mark for latest CGM with Dexcom still in the wings
The step is the latest in the rivalry between the two big players in the continuous glucose monitoring market, which Wall Street analysts contend leaves room for both to gain.
By Maria Rachal • Sept. 28, 2020 -
FDA finalizes overhauled guidance on device conformity testing pilot
The revised outline of the program, which will allow accredited labs to carry out premarket testing of medical devices, includes an expanded explanation of what the pilot means for device manufacturers.
By Nick Paul Taylor • Sept. 25, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
FDA grants EUA to first point-of-care COVID-19 antibody test
The lateral flow assay from Assure Tech achieved positive and negative percent agreements of 100% for IgG and IgM antibodies combined.
By Nick Paul Taylor • Sept. 24, 2020 -
FDA launches years-in-the-making digital health center
The center's focus areas will include the Pre-Cert program, AI and machine learning in software as a medical device, as well as cybersecurity and wireless medical devices.
By Susan Kelly • Sept. 23, 2020 -
Medtronic gets FDA breakthrough tag for device to prevent infections
The medtech giant has identified Tyrx as among the product lines to benefit from COVID-19.
By Nick Paul Taylor • Sept. 22, 2020 -
FDA De Novo OKs outpace 2019 with Spineology device, two others
A flurry of first-of-their-kind marketing authorizations comes as the agency may be receiving fewer new submissions compared to previous years, according to a MDUFA quarterly report.
By Susan Kelly • Sept. 22, 2020 -
ACLA pushes back ahead of MedPAC's 2021 report to Congress on lab fee rates
The trade group for LabCorp and Quest is wary that the Commission's rate recommendations due in June could end up being overly burdensome and not reflective of the market for all lab services.
By Greg Slabodkin • Sept. 22, 2020 -
CMS to push back radiation oncology model after industry blowback
Agency administrator Seema Verma said a six-month delay will be formalized via upcoming rulemaking because "more time is needed to prepare."
By Susan Kelly • Updated Oct. 22, 2020 -
Trump admin unveils final kidney care payment model
The CMS policy seeks to incentivize more at-home dialysis treatment, which could have implications for device makers including Baxter and newly public Outset Medical.
By Samantha Liss • Sept. 18, 2020 -
PerkinElmer tops FDA COVID-19 test sensitivity list, with BD and Roche scoring lower
PerkinElmer’s coronavirus nucleic acid kit achieved the lowest limit of detection, indicating greater sensitivity or ability to accurately detect the virus, in an agency comparison of 58 tests.
By Nick Paul Taylor • Sept. 18, 2020 -
Nixed Philips, Hamilton, Vyaire ventilator contracts subject of expanding House probe
The oversight subcommittee wants to know if the HHS cancellation of "wasteful" contracts with the three medtechs included generous termination settlements involving payoffs to the companies.
By Greg Slabodkin • Sept. 17, 2020 -
Roche gets FDA nod for faster combo coronavirus-flu test
The diagnostic, designed to detect and differentiate SARS-CoV-2 and influenzas A and B, gives results within 20 minutes, compared to a prior authorized version for a Roche system with an hours-long turnaround.
By Greg Slabodkin • Sept. 16, 2020 -
FDA finalizes voluntary consensus standard guidance
The final guidance offers updated advice for submitting requests for recognition in the premarket review process and explains when the agency may withdraw a previously recognized standard.
By Susan Kelly • Sept. 15, 2020 -
FDA touts lessons from Pre-Cert, though next steps murky
Apple, Fitbit and Johnson & Johnson are among nine companies in the pilot launched in 2019 to fast-track digital health products to market. But there's no end in sight as tests continue.
By Greg Slabodkin • Sept. 15, 2020 -
Another BD Alaris recall pegged Class I event by FDA
It's the latest in a flurry of notifications about the infusion pump line of products, which have been the subject of 18 of the high-risk recall notices since March.
By Nick Paul Taylor • Sept. 15, 2020 -
FDA AI-machine learning strategy remains work in progress
Nearly 18 months since the agency's discussion paper on a proposed regulatory framework, critical pieces are still needed for the development of draft guidance, agency digital health chief Bakul Patel said.
By Greg Slabodkin • Sept. 14, 2020 -
FDA advisers support down-classing bone growth stimulators, in face of industry and lawmaker pushback
But the orthopaedic devices panelists said there should be rigorous clinical evidence standards for the class of products currently sold by Orthofix, Zimmer Biomet, Bioventus and DJO, which are yet to be defined.
By Maria Rachal • Sept. 11, 2020 -
FDA aims to harmonize adverse event reporting with eMDR updates, makes patient problem codes public
The agency finalized adding new elements and fields to its Electronic Medical Device Reporting system, and also clarified protocols for manufacturers with EUAs or following COVID-19 enforcement policies.
By Nick Paul Taylor • Sept. 10, 2020 -
Medtronic tricuspid valve replacement device wins FDA breakthrough status
Although the medtech's Intrepid device is just starting an early feasibility study, it may ultimately go up against transcatheter tricuspid valve repair options from Abbott and Edwards.
By Maria Rachal • Sept. 9, 2020 -
Ortho device reclassifications, MDUFA reauthorization among FDA's fall meeting lineup
The agency's upcoming slate of virtual medtech meetings kicks off Tuesday with an orthopaedic devices panel that will consider a proposal to downclassify noninvasive bone growth stimulators.
By Susan Kelly • Sept. 8, 2020 -
Edwards gets FDA approval for Sapien 3 in new patient population
The PMA expands the transcatheter valve for use in congenital heart defect patients who suffer problems following surgical repair, positioning the device to compete against a range of treatment options.
By Nick Paul Taylor • Sept. 8, 2020 -
Roche COVID-19, flu combo test gets FDA emergency authorization
It is the fourth EUA for a diagnostic that detects and differentiates the viruses. However, the company claims to be the first commercial test that runs on fully automated high-throughput systems using a single sample.
By Greg Slabodkin • Sept. 4, 2020 -
FDA flags 428 spinal cord stimulator patient deaths, urges more tests before implant
The agency revealed the figures in a letter to healthcare providers urging simulations prior to permanently implanting the devices, sold by companies such as Abbott, Boston Scientific, Medtronic and Nevro.
By Nick Paul Taylor • Sept. 4, 2020 -
Watchdog gives high marks to CMS competitive bidding program
The Office of Inspector General probe found the agency followed procedures 97% of the time when awarding contracts for durable medical equipment, prosthetics, orthotics and supplies.
By Nick Paul Taylor • Sept. 3, 2020 -
Final inpatient payment rule confirms price transparency push
The American Hospital Association quickly criticized the CMS decision to move forward with collecting data on hospital median payer-specific negotiated charges to guide relative Medicare payment rates.
By Hailey Mensik • Updated Sept. 3, 2020