FDA: Page 27
-
Roche wins FDA approval for companion diagnostic to support breast cancer therapy
The approval positions Roche to support the rollout of AstraZeneca and Daiichi Sankyo’s Enhertu in HER2-low breast cancer.
By Nick Paul Taylor • Oct. 5, 2022 -
Illumina looks to resolve Grail acquisition ‘sooner rather than later’: analysts
With a divestiture order from the European Commission looming, Illumina is looking at strategic options for the maker of blood tests used to detect cancer.
By Elise Reuter • Oct. 4, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
LivaNova recall of open-heart-surgery blood pump designated as Class I event by FDA
A software malfunction can mistakenly freeze the machine, set off an alarm that can’t be muted and lead to the heart pump stopping during surgery.
By Nick Paul Taylor • Updated Oct. 12, 2022 -
Medtech groups praise MDUFA passage as legislators plan to include reforms in end-of-year bill
The legislation will provide the FDA with as much as $1.9 billion over the next five years, but changes to diagnostics and medical device cybersecurity were slashed from a "clean version" of the bill.
By Elise Reuter • Oct. 3, 2022 -
European AI Act could have ‘significant impact’ on manufacturers, medtech group warns
“Overregulation and misalignment” could create uncertainty and stop products from coming to market, MedTech Europe says.
By Nick Paul Taylor • Oct. 3, 2022 -
Pulse oximeter bias delayed treatment of Black COVID-19 patients by hours: study
An author of the paper called for manufacturers to “go back to the drawing board to provide clinicians with a tool that is free from bias.”
By Nick Paul Taylor • Sept. 30, 2022 -
Veterans Affairs, FDA partner to accelerate medical device development
The goal is to provide standardized, off-the-shelf tests that can streamline the regulatory process and reduce time to market.
By Nick Paul Taylor • Sept. 30, 2022 -
Congress passes continuing resolution reauthorizing FDA user fees; bill heads to Biden for signature
President Biden is expected to sign last-minute deal to fund the government, that includes a “clean” bill without amendments to fund FDA review activities for five years.
By Elise Reuter • Updated Sept. 30, 2022 -
FDA finalizes guidance on how clinical decision support software is regulated
The FDA has overhauled the 2019 draft, deleting some sections and completely rewriting other parts of the document.
By Nick Paul Taylor • Sept. 28, 2022 -
FDA switches COVID-19 tests to De Novo, 510(k) pathways, limiting EUAs
The FDA will still review EUA requests from “experienced developers” of tests that meet certain criteria.
By Nick Paul Taylor • Sept. 28, 2022 -
FDA user fees package won’t include any amendments, House leaders say
Cybersecurity and clinical trial diversity amendments passed by the U.S. House of Representatives won’t be in the final version of the bill.
By Elise Reuter • Sept. 27, 2022 -
FDA makes case for ‘new regulatory paradigm’ amid hurdles in software-oversight program
The agency seeks to tailor requirements based on the latest science, the benefits and risks posed by devices, their real-world performance and their contribution to promoting health equity.
By Nick Paul Taylor • Sept. 27, 2022 -
Puerto Rico-based lab ceases COVID-19 test; FDA revokes EUA
The FDA disclosed the action alongside updates to the instructions for use for Thermo Fisher Scientific’s TaqPath COVID-19 test.
By Nick Paul Taylor • Sept. 26, 2022 -
Philips’ latest BiPAP machine recall labeled Class I event by FDA
Certain bi-level positive airway pressure devices feature plastic that could cause machines to stop working suddenly, potentially leading to serious injury or death, the FDA said Friday.
By Nick Paul Taylor • Sept. 26, 2022 -
Device makers with ethylene oxide facilities at risk of lawsuits after Sterigenics loss: Needham
The $363 million award in the Sterigenics lawsuit “could lead to increased aggressiveness on the part of attorneys,” Needham analysts warned in a Friday note to investors.
By Ricky Zipp • Sept. 23, 2022 -
FDA user fee package to be included in Congress’ bill to fund government, avoid shutdown, Senators say
A “practically clean” version of the bill will allow the FDA to continue its review and testing programs, while leaving more ambitious goals to a funding bill later this year.
By Elise Reuter • Sept. 22, 2022 -
As deadline for FDA user fees bill nears, Congress weighs continuing resolution
Reauthorization bill is likely to pass before Sept. 30, but it’s unclear what provisions will be included in the final version, experts said.
By Elise Reuter • Updated Sept. 22, 2022 -
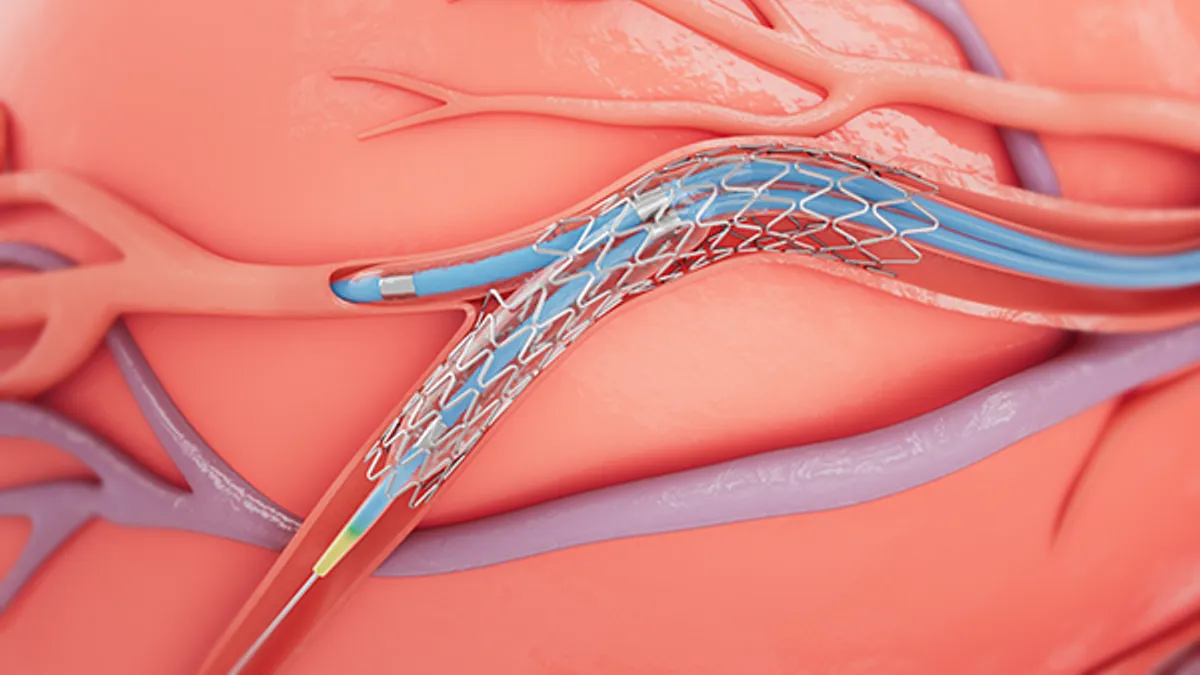
 Courtesy of https://filecache.mediaroom.com/mr5mr_medtronic/183509/SURTAVI%20%28002%29%209.21%20600pix.png
Courtesy of https://filecache.mediaroom.com/mr5mr_medtronic/183509/SURTAVI%20%28002%29%209.21%20600pix.png
Medtronic gets first FDA approval for drug-eluting stent in bifurcation lesions
Researchers have found the one-device, provisional stenting method delivers better results than two stents in some patients.
By Nick Paul Taylor • Sept. 22, 2022 -
Watchdog faults FDA for rushing COVID-19 tests to market by easing emergency use rules
HHS’ Office of Inspector General found that by loosening emergency use authorization requirements to bring COVID-19 tests to market faster, the agency allowed inaccurate tests to be distributed.
By Elise Reuter • Sept. 21, 2022 -
Insulet’s Omnipod 5 gains CE Mark for European release
After months of regulatory delays, Insulet’s newest insulin pump can now be marketed in the U.S. and European Union.
By Nick Paul Taylor • Sept. 21, 2022 -
Jury decides against Sterigenics in ethylene oxide trial, awarding $363M to the sole plaintiff
Sterigenics has more than 760 lawsuits pending against it in the local court for toxic emissions previously emitted by the plant.
By Nick Paul Taylor • Sept. 20, 2022 -
FDA updates advice about Medtronic endotracheal tubes after Class I recall, patient death reports
The FDA provided more detailed recommendations on preventing and responding to airway obstruction that may occur when using some Medtronic endotracheal tubes.
By Peter Green • Sept. 19, 2022 -
Racial bias in pulse oximeters to be subject of FDA panel
The design of the ubiquitous fingertip monitors has inadvertently caused false blood oxygen readings in darker-skinned patients, a problem the FDA hopes to fix.
By Peter Green • Sept. 16, 2022 -
Baxter’s recall of solution sets used in chemotherapy labeled Class I by FDA amid complaints of leaks
The regulator says the leaks could kill, but with 83 complaints it has not received reports of patient injuries or deaths.
By Ricky Zipp • Sept. 15, 2022 -
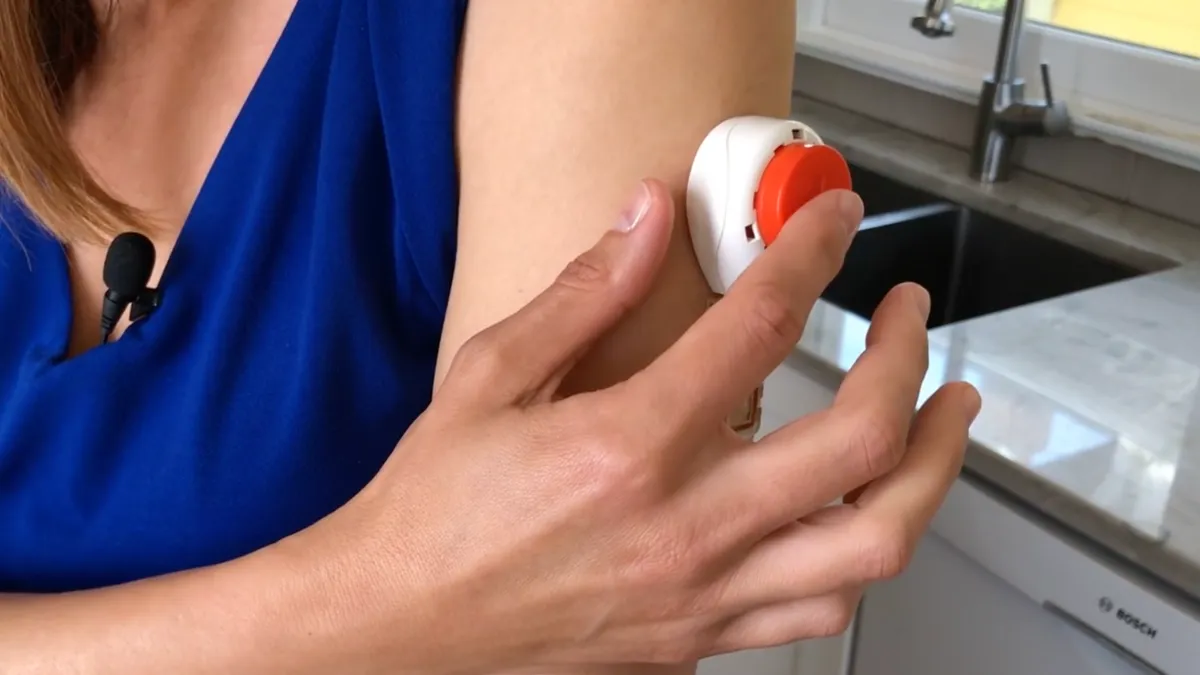
 Retrieved from https://www.tassoinc.com/product-use-videos on September 15, 2022
Retrieved from https://www.tassoinc.com/product-use-videos on September 15, 2022
Tasso’s at-home blood collection lancet wins FDA clearance
Tasso is pitching the device as an enabler of decentralized clinical trials because it could simplify the collection of blood at home.
By Nick Paul Taylor • Sept. 15, 2022