FDA: Page 2
-
Dexcom receives warning letter based on FDA inspections of 2 plants
The company does not expect the warning letter to materially impact its manufacturing capacity, sales or ability to seek clearance for new products.
By Nick Paul Taylor • March 10, 2025 -
Following FDA cuts, Trump nominee Makary vows ‘independent’ staff review
The Johns Hopkins surgeon highlighted rapid growth at the agency, but pledged key staff will have “all the resources they need to do their job well.”
By Jonathan Gardner • March 6, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Makary, under review to run FDA, evades pressure to reinstate canceled vaccine meeting
Questioned by senators at a Thursday hearing, President Donald Trump's FDA nominee said he would reevaluate which scientific topics require an advisory committee's input.
By Ned Pagliarulo • March 6, 2025 -
Trump administration policies could create headwinds across healthcare: Fitch
The credit ratings agency is most concerned about Medicaid cuts and how government layoffs will affect device approvals.
By Susanna Vogel • March 5, 2025 -
Philips pulls endovascular implant from market after 20 injuries
Philips stopped selling its Tack endovascular system because of cases where additional interventions were needed to remove the implant.
By Elise Reuter • March 5, 2025 -
FDA recall notice details J&J pause of Varipulse
Four of the 132 patients treated with Varipulse in the first two months of the U.S. launch had a form of stroke shortly after surgery.
By Nick Paul Taylor • March 3, 2025 -
Tandem’s insulin pump tech wins FDA nod for Type 2 diabetes
Tandem is following competitor Insulet’s lead with the second automated insulin delivery system indicated for Type 2 diabetes.
By Elise Reuter • Feb. 27, 2025 -
‘Fear and uncertainty’: Biotech investors warn of impact from NIH research cuts
The Trump administration’s plans to reduce NIH grant funding could have long-term consequences for the U.S. drug industry, investors said.
By Gwendolyn Wu • Feb. 27, 2025 -
CAP urges HHS to revoke lab developed test rule, citing Trump order
The pathologists group wants the regulation terminated in light of an executive order from President Trump that calls for federal agencies to “alleviate unnecessary regulatory burdens.”
By Nick Paul Taylor • Feb. 26, 2025 -
FDA brings back some fired device staff
An industry source said “most, if not all” of the people at the CDRH who were recently dismissed are now being asked to return.
By Elise Reuter • Feb. 24, 2025 -
Medtronic gains FDA OK for self-adjusting DBS system for Parkinson’s
The brain-computer interface technology personalizes therapy based on an individual’s brain activity.
By Susan Kelly • Feb. 24, 2025 -
In FDA job cuts, experts see threat of far-reaching impact
"Any place that gets cut, it's going to have an impact, because there's not any spare personnel at FDA,” said former agency commissioner Robert Califf.
By Jonathan Gardner , Delilah Alvarado • Feb. 21, 2025 -
Device industry scrambles as FDA job cuts cause delays
The cuts at the FDA’s device center could add “months, if not years” to the time it takes to bring products to market, an attorney said.
By Elise Reuter • Feb. 20, 2025 -
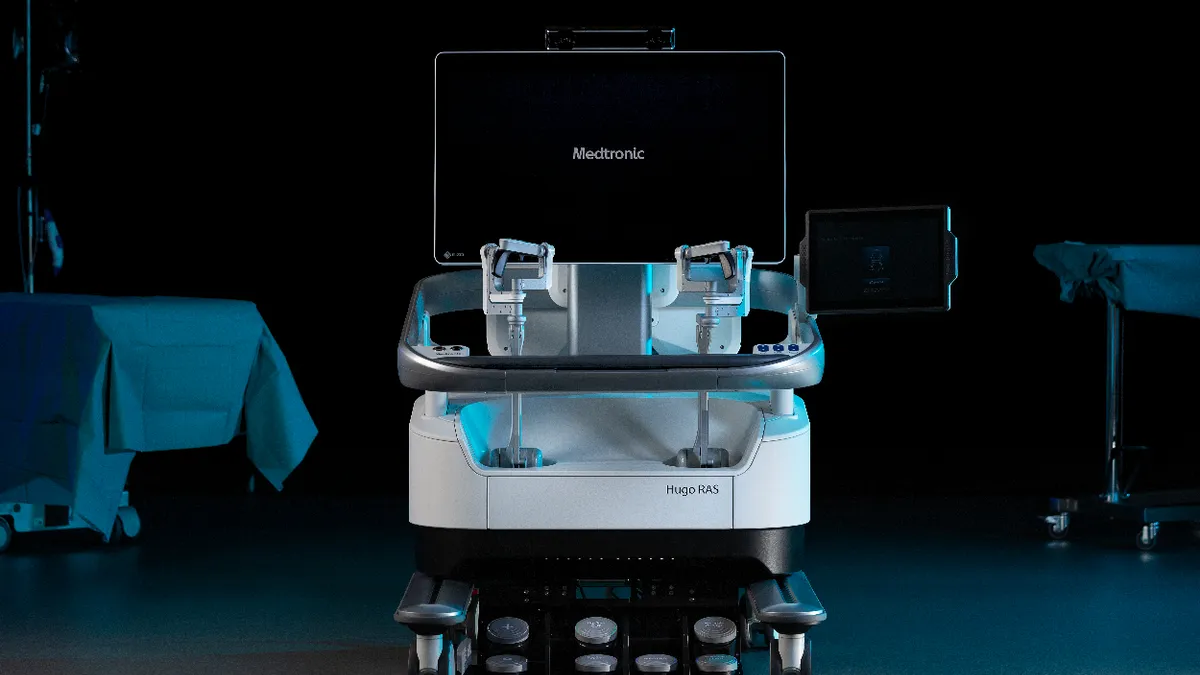
Medtronic nears FDA submission for Hugo robot
CEO Geoff Martha said Medtronic would submit its application for urological procedures to the Food and Drug Administration by the end of March.
By Ricky Zipp • Feb. 18, 2025 -
RFK Jr. confirmed as HHS secretary
Robert F. Kennedy Jr., a prominent vaccine critic, was confirmed Thursday by the U.S. Senate in a 52-48 vote to serve as the nation’s top health official.
By Emily Olsen • Feb. 13, 2025 -

 Retrieved from Screenshot: The Food and Drug Administration.
Retrieved from Screenshot: The Food and Drug Administration.
Judge orders FDA, health agencies to restore removed webpages
District Judge John Bates wrote the removals harm “everyday Americans, and most acutely, underprivileged Americans, seeking healthcare.”
By Elise Reuter • Feb. 12, 2025 -
Trump orders agencies to plan for ‘large-scale’ job cuts
While Trump’s executive order provides substantial power to Elon Musk’s DOGE effort, it’s not specifically targeted to health agencies like the FDA.
By Ned Pagliarulo • Feb. 12, 2025 -
FDA posts early alert about BD device linked to 30 injuries, 4 deaths
The alert was posted one day after BD sent customers a letter warning about the risk of atherectomy catheters breaking.
By Nick Paul Taylor • Feb. 10, 2025 -
Medtronic recall of brain fluid drainage systems linked to 15 injuries
The company will keep the systems on the market but is asking providers to check devices for visible cracks and return affected devices.
By Nick Paul Taylor • Feb. 4, 2025 -
RFK Jr. clears key hurdle on path to HHS secretary
A key Senate committee voted to advance Robert F. Kennedy’s nomination even after some legislators raised concerns about his previous vaccine stances and confusion surrounding programs like Medicare and Medicaid.
By Sydney Halleman , Delilah Alvarado , Jonathan Gardner • Feb. 4, 2025 -

 Retrieved from Olympus on February 03, 2025
Retrieved from Olympus on February 03, 2025
FDA labels recall of discontinued Olympus endoscope part as Class I
The update comes about six weeks after an early alert flagged reports of 120 injuries and one death linked to reprocessing of the device.
By Susan Kelly • Feb. 3, 2025 -
Troy Tazbaz, CDRH digital health leader, resigns
Tazbaz was director of the FDA’s Digital Health Center of Excellence for two years, leading key policies on artificial intelligence and software as a medical device.
By Elise Reuter • Jan. 31, 2025 -
2025 outlook
Top medtech trends in 2025: New products and M&A are bright spots, but Trump brings uncertainty
Experts highlighted surgical robotics, artificial intelligence and M&A as top trends this year. However, the Trump administration could create challenges for the industry.
By Ricky Zipp • Jan. 31, 2025 -
FDA warns about patient monitor cybersecurity vulnerabilities
Vulnerabilities in certain Contec and Epsimed patient monitors can allow people to gain access and potentially manipulate the devices, the FDA warned.
By Nick Paul Taylor • Jan. 31, 2025 -

 Retrieved from Screenshot: The Food and Drug Administration.
Retrieved from Screenshot: The Food and Drug Administration.
FDA webpages on clinical trial diversity removed after Trump orders
The website changes raise concerns about “the interference of politics with the study and the practice” of science and medicine, a physician said.
By Elise Reuter • Jan. 27, 2025