FDA: Page 11
-
EU launches probe of China’s medical device market
China’s procurement market for medical devices has gradually become more closed for European and foreign companies, the European Commission alleges.
By Susan Kelly • April 24, 2024 -
FTC votes to ban noncompetes, with far-reaching effects on doctors
The FTC estimates the final rule would lower healthcare costs by $194 billion over the next decade, while freeing up physicians to more easily move between employers.
By Rebecca Pifer • April 24, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Medtronic names Yarmela Pavlovic as chief regulatory officer
Pavlovic, who was a partner at the law firm Manatt, Phelps & Phillips before joining Medtronic, will gain a new title, but her role will stay the same.
By Nick Paul Taylor • April 24, 2024 -
FDA approves Lumicell’s breast cancer imaging tool
Lumicell developed the technology to enable physicians to detect residual cancer in the breast cavity after surgery.
By Nick Paul Taylor • April 22, 2024 -
Exactech recalls shoulder devices after initially declining to act
After the FDA issued a public safety notice and sent Exactech a warning letter, the firm agreed to recall shoulder implants due to packaging issues.
By Nick Paul Taylor • April 22, 2024 -
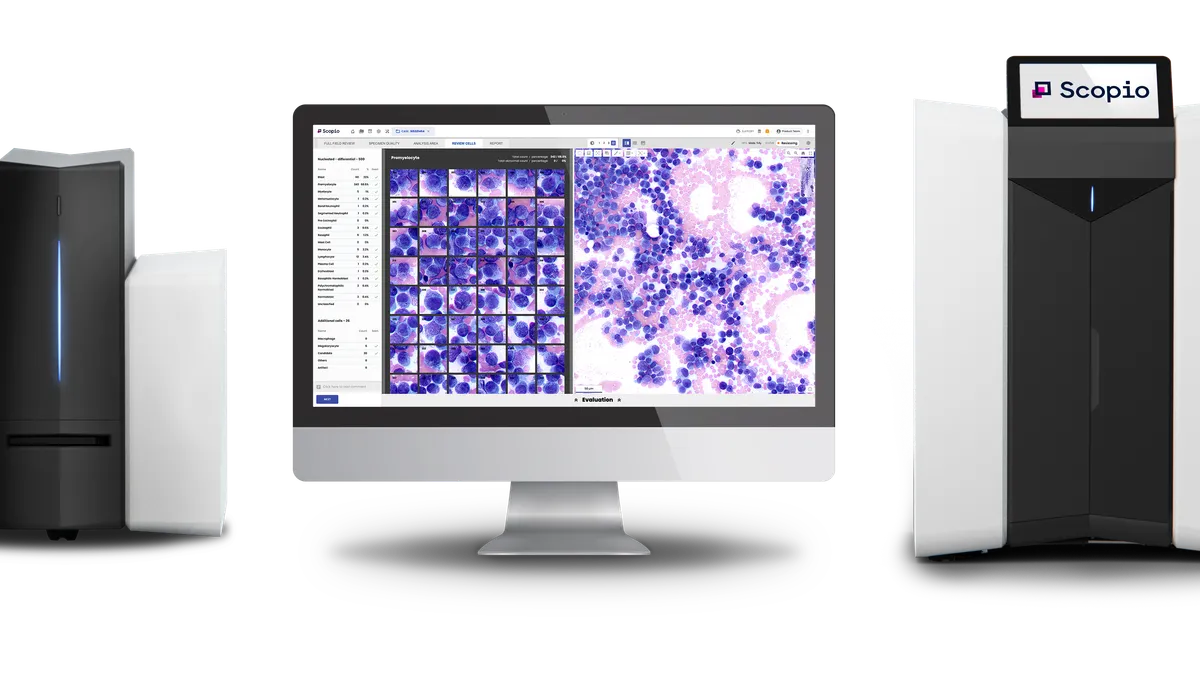
Scopio Labs wins de novo nod for bone marrow analysis software
Scopio can now add the application to its imaging platforms that allow users to view blood samples digitally rather than under a microscope.
By Nick Paul Taylor • April 18, 2024 -
Boston Scientific recalls blood-blocking agent linked to 2 deaths
Seven injuries and 11 incidents were also associated with the safety issue.
By Nick Paul Taylor • April 18, 2024 -
‘An incredible undertaking’: 5 takeaways from Philips consent decree
Under the agreement, the FDA will use rare powers to require repairs, replacements or refunds for recalled respiratory machines.
By Elise Reuter • Updated April 17, 2024 -
FDA warns Soulaire to stop selling device outside authorized uses
Soulaire marketed its external counterpulsation system to grow new arteries, repair organ dysfunction and treat COVID-19, among other uses, without evidence.
By Nick Paul Taylor • April 17, 2024 -
European Commission approves Illumina’s proposed Grail split
Selling Grail to a third party would free Illumina of the need to capitalize the company, but the buyer would need the commission’s approval, analysts wrote.
By Nick Paul Taylor • April 15, 2024 -
FDA expands import alert to block all plastic syringes from Chinese manufacturer
The agency increased the blockade because Jiangsu Shenli Medical Production failed to meet device quality system requirements.
By Nick Paul Taylor • April 11, 2024 -
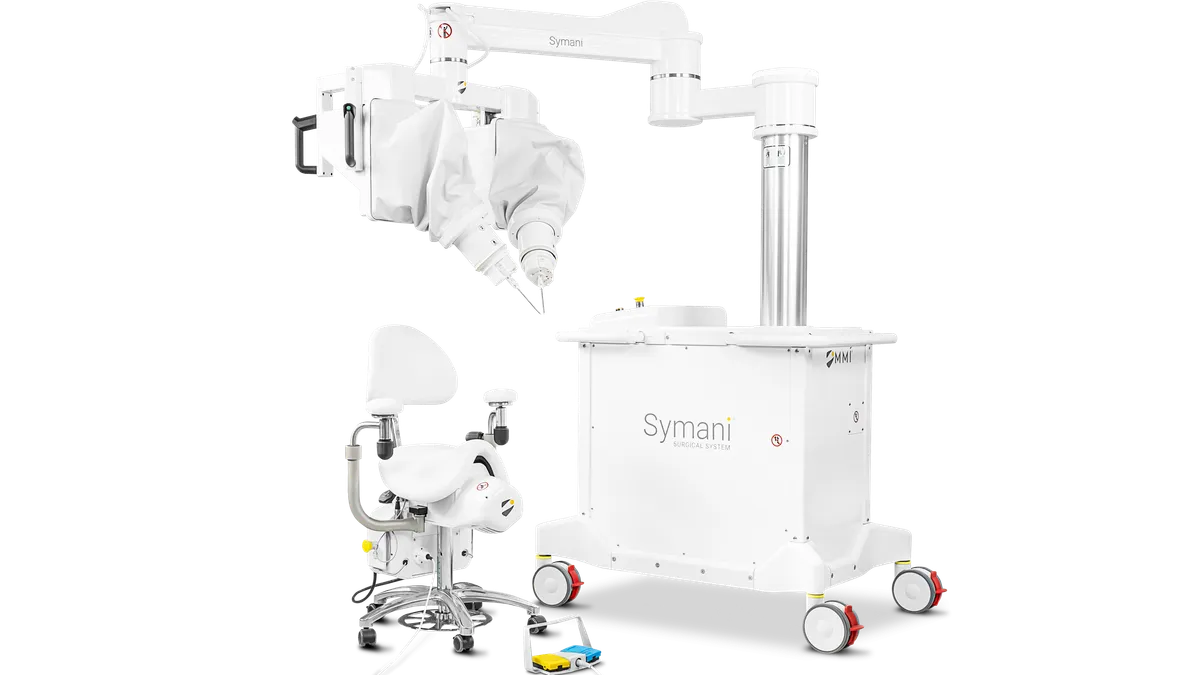
MMI receives de novo nod for microsurgery robot
Medical Microinstruments said the system could increase the number of physicians who can perform complicated microsurgical procedures.
By Nick Paul Taylor • April 9, 2024 -
Physicians ask FDA to revoke approval of DNA test for opioid addiction
Physicians said the test is based “on old genetic studies that have largely been abandoned” and could exacerbate the opioid crisis.
By Nick Paul Taylor • April 8, 2024 -
Beckman Coulter receives FDA warning letter
Inspectors found fault with quality practices at a facility that makes immunoassay analyzer instruments and tests.
By Nick Paul Taylor • April 5, 2024 -
Smiths Medical recalls thousands of ventilators over fault linked to 8 serious injuries
A problem with the devices can cause patients to receive the wrong amount of ventilation or too little oxygen, the FDA said in a recall notice.
By Nick Paul Taylor • April 5, 2024 -
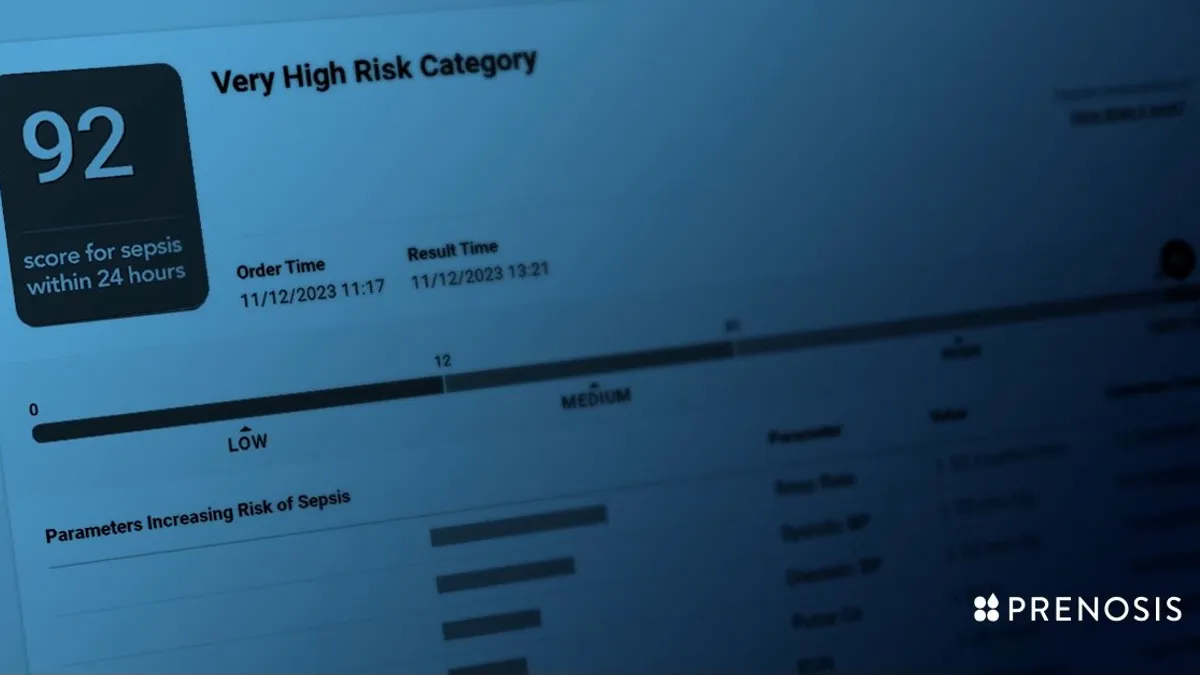
FDA grants de novo nod to AI tool for detecting sepsis
Prenosis CEO Bobby Reddy Jr. told MedTech Dive the company sees third-party validation as important, with the FDA having clarified that certain decision support tools should be regulated as medical devices.
By Elise Reuter • April 4, 2024 -

 Retrieved from Axonics on August 22, 2023
Retrieved from Axonics on August 22, 2023
FTC asks Boston Scientific for more info on $3.7B Axonics deal
The company has pushed back its expected closing date for the acquisition due to the second request from the Federal Trade Commission.
By Susan Kelly • April 4, 2024 -
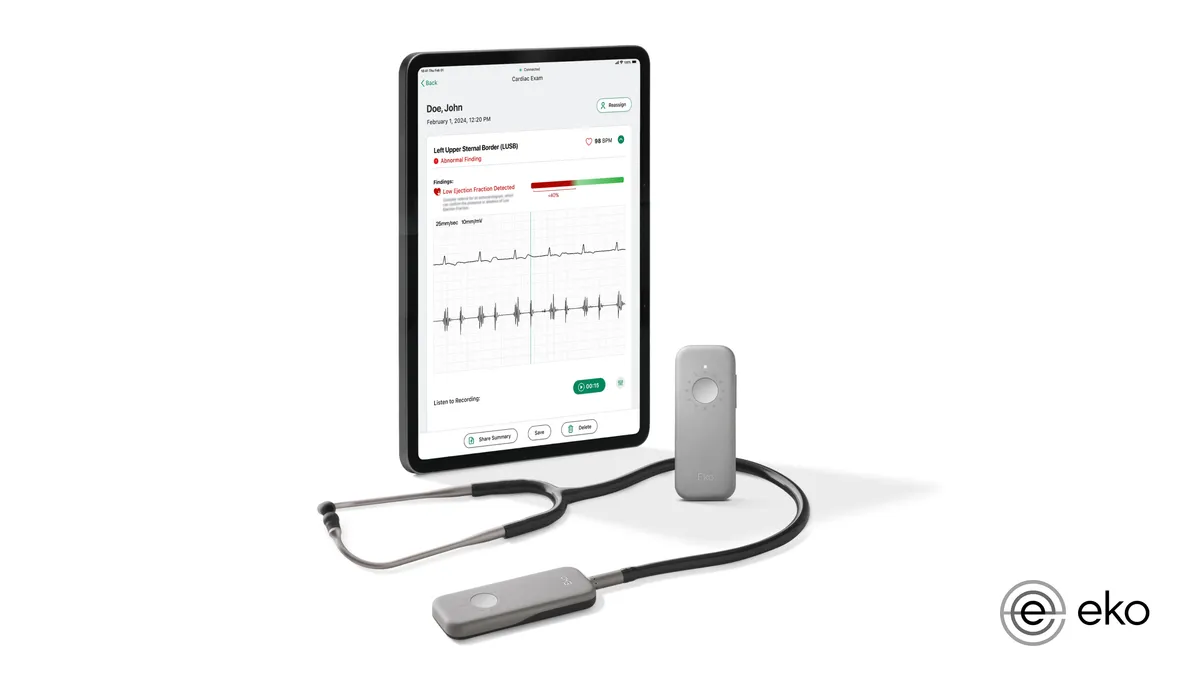
Eko wins FDA nod for AI to detect sign of heart failure using stethoscope
The clearance adds to the list of devices the FDA has authorized this year with AI algorithms to detect health conditions.
By Nick Paul Taylor • April 4, 2024 -
Teleflex catheterization kit recall linked to 10 injuries, 1 death
Nearly 335,000 devices were recalled due to safety issues, which may cause damage to blood vessel walls, artery blockage or death.
By Nick Paul Taylor • April 4, 2024 -
Deep Dive
As cyberattacks on healthcare persist, can the FDA’s new device regs hold up?
Revamped regulations to thwart hackers are a big step forward, but issues such as legacy devices and reliance on software patches pose lingering challenges.
By Ricky Zipp • April 3, 2024 -
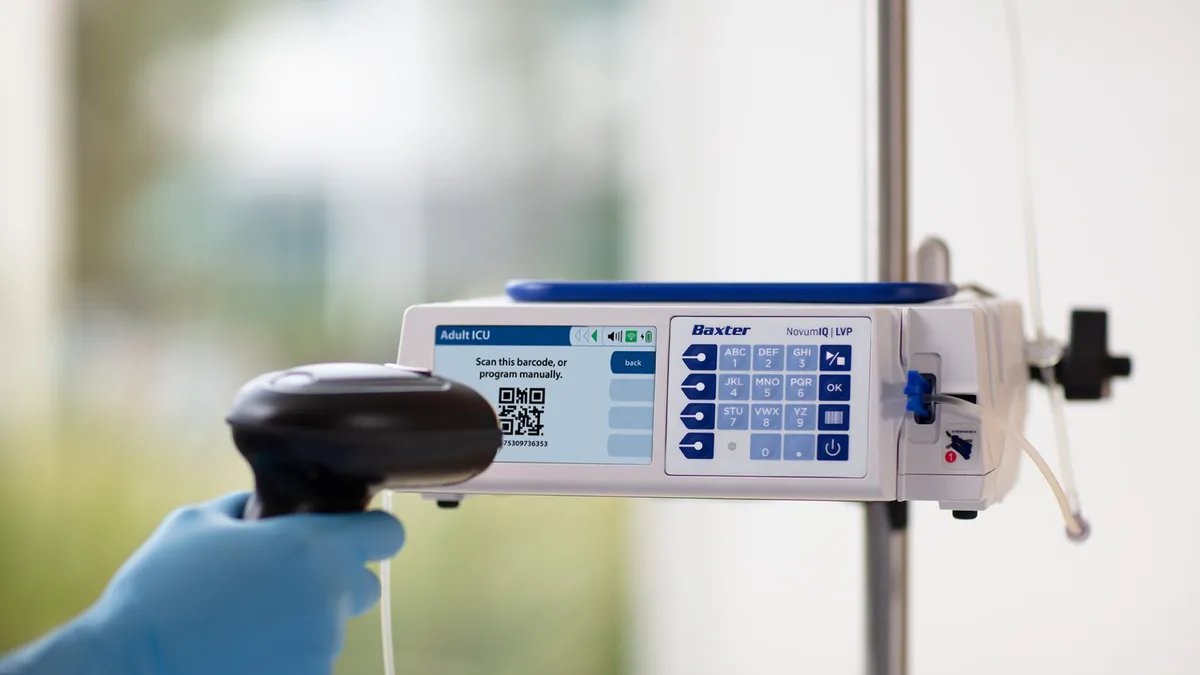
Baxter receives FDA clearance for delayed Novum IQ infusion pump
The clearance ends a three-year back-and-forth with the FDA to get the product to market.
By Nick Paul Taylor • April 2, 2024 -
Abbott wins FDA clearance for bedside blood concussion test
The clearance is a step towards using the test in non-healthcare settings, such as sporting events, Abbott said.
By Nick Paul Taylor • April 2, 2024 -
Infutronix pulls infusion pumps from US after nearly 3,700 complaints
The company listed six issues that can cause the pumps to stop infusions and otherwise malfunction.
By Nick Paul Taylor • April 2, 2024 -
Roche wins FDA approval for first molecular malaria blood donor screening test
The company is pitching the test as a way to improve the safety and availability of blood.
By Nick Paul Taylor • March 28, 2024 -
FDA hits Renovo with warning letter over reprocessed medical devices
The letter accuses Renovo of adding models other than the ones covered by its 510(k) clearances.
By Nick Paul Taylor • March 27, 2024