Medical Devices: Page 74
-
Abbott gets FDA breakthrough tag for deep brain stimulation system in depression
The breakthrough device designation builds on a series of clinical trials of other systems that have yielded mixed results.
By Nick Paul Taylor • July 13, 2022 -
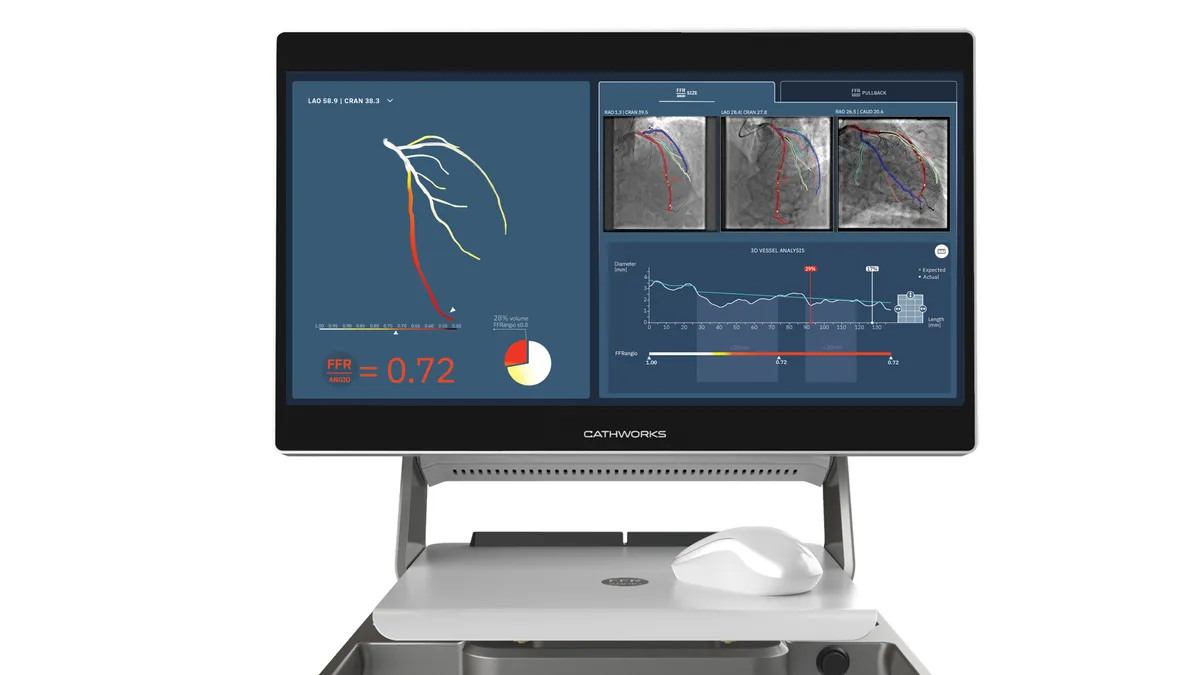
Medtronic lands option to buy artery disease player CathWorks for up to $585M
CathWorks has the right to compel Medtronic to complete the acquisition if it chooses not to exercise its option.
By Nick Paul Taylor • July 13, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Faulty oximeter readings led to racial, ethnic disparities in treatment: study
Researchers previously noted that the devices may be less accurate when used by people with darker skin, and a recent study found the difference led to patients failing to receive needed treatment.
By Elise Reuter • July 12, 2022 -
CMS makes 'market-moving proposals' to some medtech Medicare rates, say analysts
BTIG analysts warned in a recent report that the changes “might curtail usage of some higher-dollar” medical devices.
By Nick Paul Taylor • July 12, 2022 -
European Commission targets spring of 2024 for fully functional Eudamed database
The Commission is planning a two-phase transition to mandatory use of the database after it goes live.
By Nick Paul Taylor • July 11, 2022 -
Medtronic recalls over 1M dialysis catheters due to malfunction
The recall is the latest in a growing list of product safety issues for Medtronic, including Class I recalls and an FDA warning letter for its diabetes unit.
By Ricky Zipp • July 11, 2022 -
Ex-Mazor executive charged by SEC with insider trading over Medtronic's $1.6B buyout
Doron Tavlin, who was Mazor’s vice president of business development, is accused by the agency of profiting from his inside knowledge of the pending takeover.
By Nick Paul Taylor • July 11, 2022 -
Q&A
Friday Q&A: Roy Jakobs, Philips' head of connected care, talks sleep apnea recall, changing safety protocols
In the midst of a recall of 5.5 million sleep apnea devices and ventilators, Jakobs spoke about the company's recall process, communication efforts, and how the repair and replacement program is progressing.
By Ricky Zipp • July 8, 2022 -
CMS proposes national rates for cardiac monitoring; iRhythm's stock jumps
The proposed rule could end a years-long saga for the cardiac market, although pricing specifics may change in the final version.
By Ricky Zipp • July 8, 2022 -
Semiconductor shortage leaves medtech industry 'more pessimistic' as customers leave, says Deloitte
Hospitals and health systems are looking into alternative products as a result of the disruption, according to a report from Deloitte and AdvaMed.
By Nick Paul Taylor • July 7, 2022 -
Getinge recalls anesthesia machines due to cracked, broken suction power switches
The FDA labeled the recall a Class I event, marking Getinge’s fifth Class I recall since September.
By Ricky Zipp • July 7, 2022 -
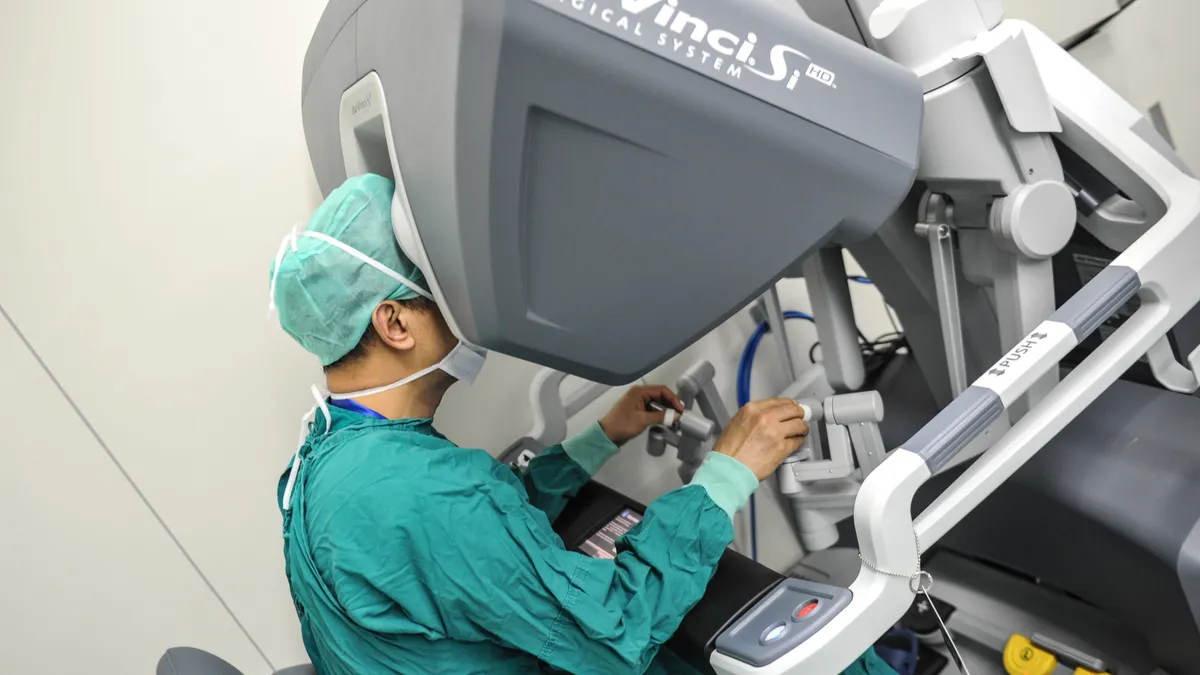
Intuitive's ecosystem is making it harder for J&J, Medtronic to crack robotic surgery market, say analysts
Intuitive’s competitors will need an “ecosystem approach” that goes beyond hardware to make it easier for customers to switch, say analysts from BTIG.
By Nick Paul Taylor • July 7, 2022 -
Wearables are a growing part of doctor-patient talks for cardiac patients: study
As mentions of wearables in primary care grew, Apple increasingly replaced Fitbit as the most-discussed wearable brand in talks between physicians and patients in the study, which covered the years 2005 to 2019.
By Nick Paul Taylor • July 6, 2022 -
Senators press FDA to finalize OTC hearing aids, accuse industry of 'astroturf' campaigns
The two cross-aisle politicians said they want to “expand access, reduce costs, and ensure a robust new market for safe and effective OTC hearing aids” and accused the device manufacturing lobby of “harming American consumers.”
By Elise Reuter • July 6, 2022 -
'Little sign' that digital tools are being deployed effectively in fight against COVID outbreak, study finds
To help reduce the spread of the next pandemic, there is “a substantial practical advantage” to integrating the features of multiple smartphone apps, a Scripps Research Translational Institute team found.
By Nick Paul Taylor • July 6, 2022 -
American Contract Systems' COVID-19 test recall gets Class I label from FDA
Off-site assembly by workers who may not have been properly trained prompted the company to recall COVID-19 tests amid concerns they may yield false results.
By Nick Paul Taylor • Updated July 6, 2022 -
AliveCor ECG patent ruling sets stage for block on Apple Watch imports
Apple Watch imports to the U.S. could be barred if ruling by International Trade Court judge is finalized; Judge says Apple Watch infringes two cardiogram patents.
By Nick Paul Taylor • July 5, 2022 -
Q&A
Friday Q&A: Canary Medical CEO Bill Hunter discusses knee-implant sensors, device reimbursement
Canary’s founder discusses how the firm plans to create predictive tools from data collected by the devices, and long-term plans to embed its sensors in other implants.
By Elise Reuter • July 1, 2022 -

 Retrieved from LiveMetric website on July 01, 2022
Retrieved from LiveMetric website on July 01, 2022
LiveMetric's blood-pressure 'smartwatch' device gets FDA clearance
The wristwatch-like device will be made available through health systems, insurers and self-insured employers to ease continuous monitoring of blood pressure and avoid white-coat syndrome.
By Nick Paul Taylor • July 1, 2022 -
 Retrieved from Siemsens Website on July 01, 2022
Retrieved from Siemsens Website on July 01, 2022
Intuitive secures FDA clearance for lung biopsy robot featuring Siemens' imaging tech
The FDA clearance is an “incremental positive” for Intuitive, wrote analysts at RBC Capital Markets, adding that they see Ion as “an important leg of growth for the company.”
By Nick Paul Taylor • July 1, 2022 -
As Philips works to replace or repair recalled devices, supply problems are slowing it down
Logistical challenges mean patients won’t know when they will get a replacement sleep apnea or ventilator device until “at best a few weeks before it is delivered,” Philips executives said.
By Ricky Zipp • June 30, 2022 -
GE Healthcare powers Cleveland Clinic imaging spinout to $33M financing round
Centerline will use the funding to expand into Europe and other international markets next year, as well as to advance the use of its technology in new surgical applications.
By Nick Paul Taylor • June 30, 2022 -
Eargo reaches financing deal as it looks to regain insurance coverage after DOJ settlement
A $125 million financing deal puts pressure on the hearing aid maker to make a quick turnaround as it works to win back its largest customer, the Federal Employee Health Benefits Program.
By Elise Reuter • June 29, 2022 -
Deep Dive
Companies see a future for smart implants. Doctors are waiting for proof.
Zimmer Biomet and Canary Medical are rolling out the first knee implant with an integrated sensor, though it will take time to learn how to incorporate that data into patient care, experts say.
By Elise Reuter • June 28, 2022 -
Sleep apnea devices show 'very low' amount of degraded foam, Philips says
Philips found evidence of foam breaking down in about 2% of 60,847 returned first generation DreamStation devices. The company said the problem was more prevalent in devices that used an ozone cleaning method.
By Ricky Zipp • June 28, 2022