Medical Devices: Page 62
-
Click wins FDA breakthrough designation for prescription digital migraine therapy
The designation would give Click Therapeutics expedited review of its preventive treatment for episodic migraine in adults.
By Nick Paul Taylor • Dec. 19, 2022 -
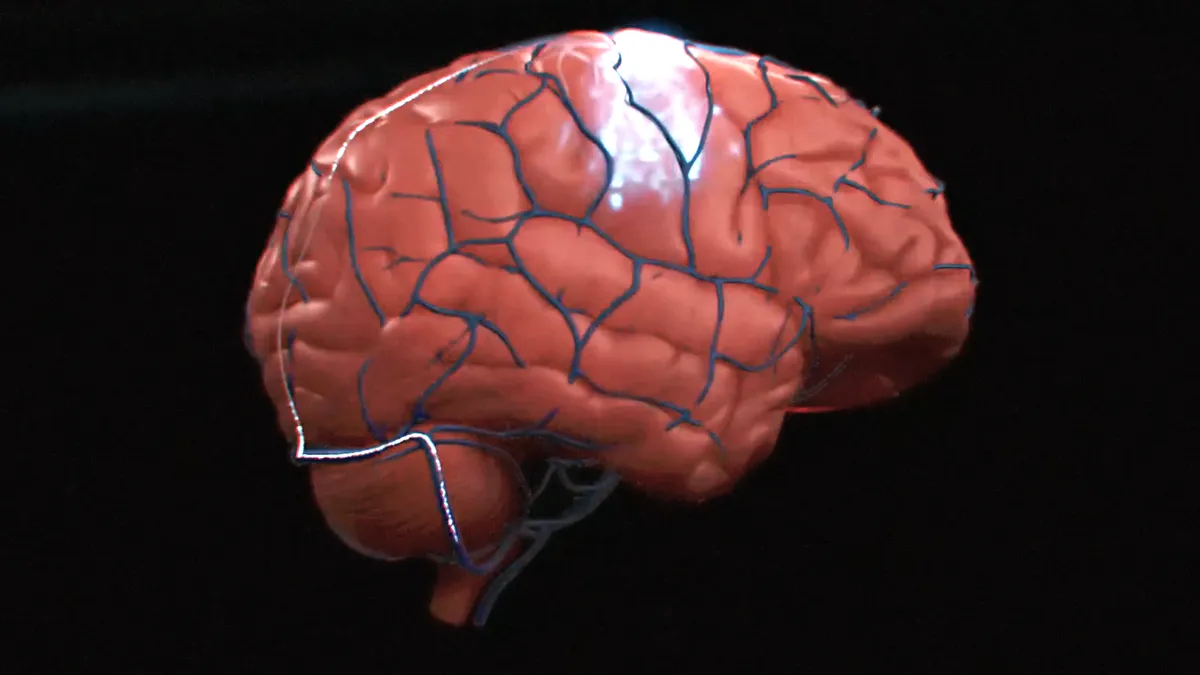
Gates, Bezos invest in Synchron’s brain computer interface
A $75 million investment will accelerate the development of a drill-free implant to help patients with upper-limb paralysis.
By Peter Green • Dec. 16, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Siemens, GE Healthcare are interested in Medtronic spinoff units: Bloomberg
Medtronic said in October it plans to divest its patient monitoring and respiratory interventions businesses.
By Elise Reuter • Dec. 15, 2022 -
Masimo links passive health monitors to home stereos amid legal row with Apple
The move integrates the consumer audio brands Masimo bought in April with its wearable health monitors.
By Peter Green • Dec. 15, 2022 -
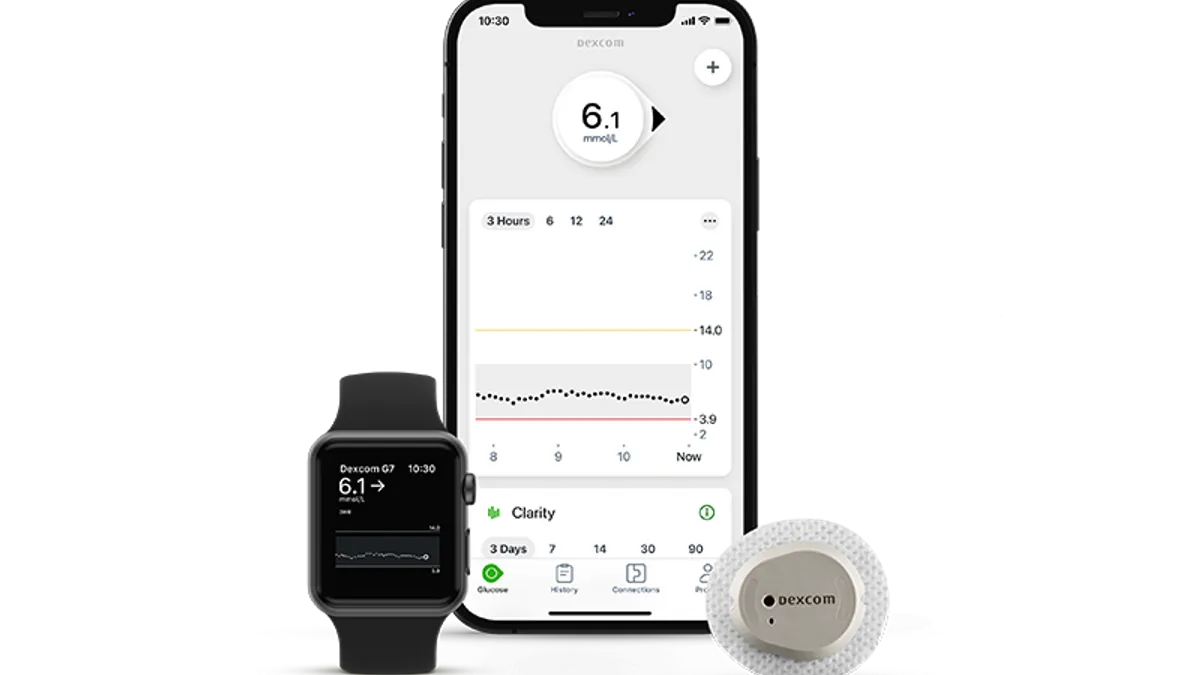
Dexcom’s Jake Leach discusses preparations for G7 launch next year
The diabetes tech company is in conversations with payers and is opening a new manufacturing plant ahead of the planned launch of its newest CGM, the COO said.
By Elise Reuter • Dec. 15, 2022 -
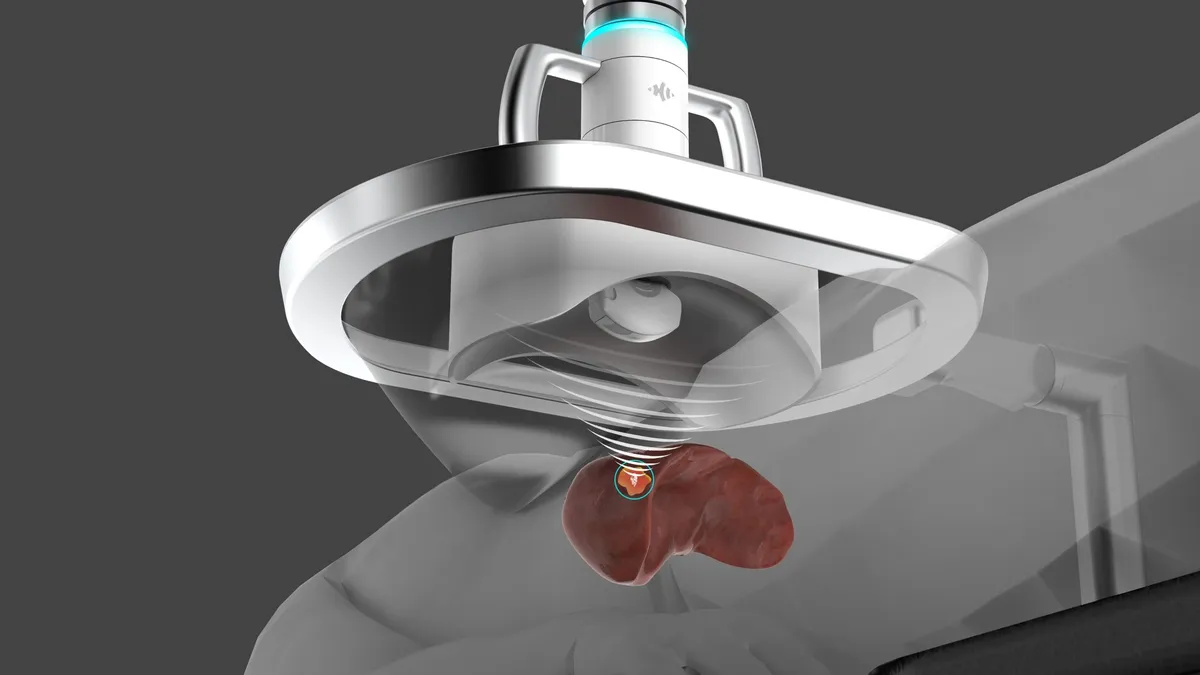
J&J leads $85M investment in HistoSonics ahead of liver cancer device authorization
HistoSonics is developing a device that delivers focused ultrasound to destroy and liquefy unwanted tissue and tumors without the need for surgical incisions.
By Nick Paul Taylor • Dec. 14, 2022 -
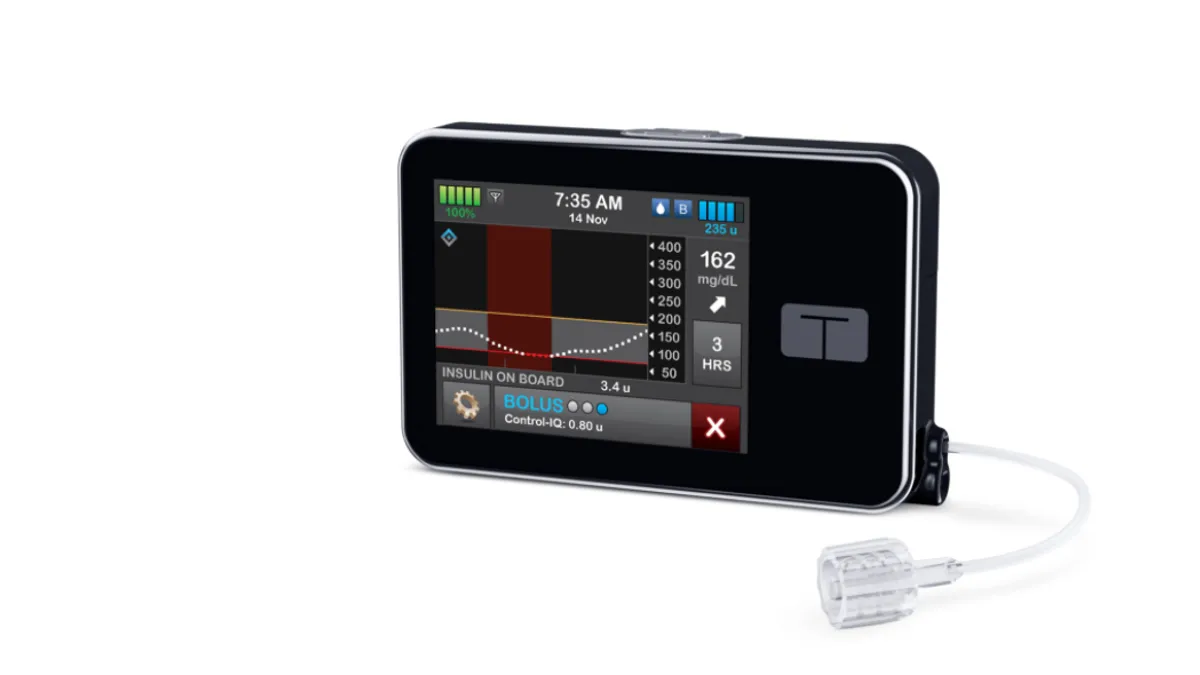
Tandem agrees to buy AMF, challenging Insulet for insulin pump patch market
The deal will give Tandem control of Sigi, a reusable, rechargeable patch pump that is designed to be smaller and lighter than existing devices.
By Nick Paul Taylor • Dec. 14, 2022 -
Deep Dive
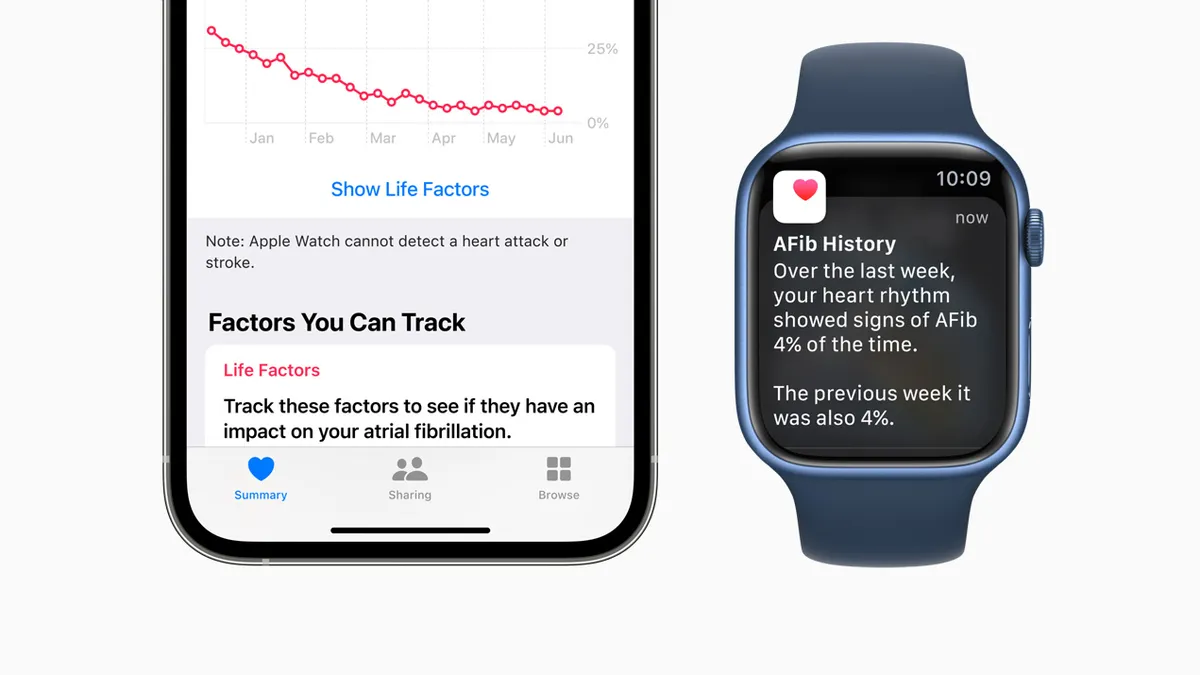
Apple locked in court battles with small medtech firms over allegations of stolen technology
AliveCor and Masimo are looking to bar Apple from selling watches that use technology the firms claim they invented.
By Elise Reuter , Nick Paul Taylor • Updated Dec. 13, 2022 -
Boston Scientific pays $523M for control of Chinese device maker Acotec
The acquisition will give Boston greater access to the Chinese market, which the U.S. firm expects will account for 25% of the global medtech demand by 2030.
By Peter Green • Dec. 12, 2022 -
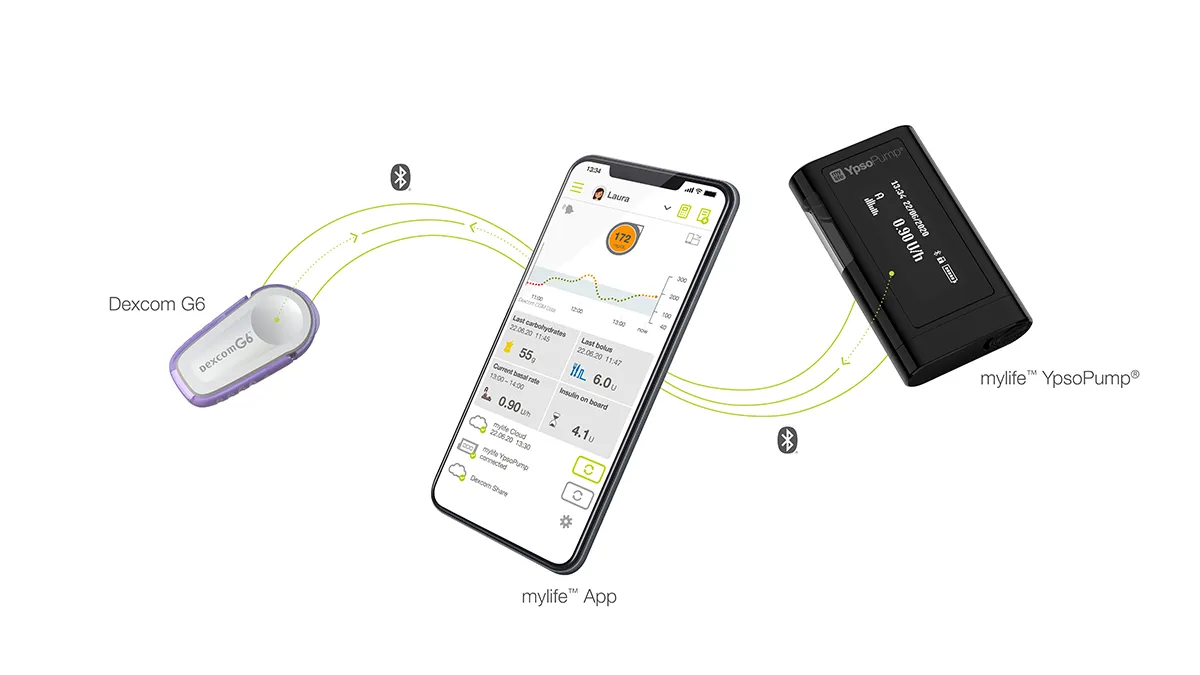
Ypsomed seeks new partner for US insulin pump launch as Lilly quits deal
The companies planned to launch a customized version of the YpsoPump that used pre-filled insulin cartridges for Lilly’s rapid-acting insulins.
By Nick Paul Taylor • Dec. 12, 2022 -
EU Health Commissioner proposes MDR delay to prevent medical device shortages
Plans to delay MDR certification to 2027 for high-risk devices and 2028 for others may still not be enough to stave off shortages.
By Nick Paul Taylor • Updated Dec. 23, 2022 -
Investors hand Vaxxas $23M to support trial of needle-free COVID-19 vaccine
With support from the Gates Foundation, the needle-free technology could get more people vaccinated and avoid cold-chain issues.
By Nick Paul Taylor • Dec. 12, 2022 -
Q&A
Friday Q&A: GE Healthcare’s Arduini, Zodl talk about spinoff, M&A, margins, Jack Welch
The CEO and CFO of the company spoke with MedTech Dive just weeks before the healthcare business formally separates from General Electric.
By Peter Green • Dec. 9, 2022 -
Chinese market recovery could spur international medtech growth in 2023: Goldman
A gradual return to normal and a new government quota could ramp up medical device sales in China later in 2023, the analysts wrote.
By Nick Paul Taylor • Dec. 9, 2022 -
Cochlear’s $120M purchase of hearing-implant maker stirs European scrutiny
The European Commission said the merger “threatens to significantly affect competition in the market for cochlear implants and bone conduction solutions.”
By Nick Paul Taylor • Dec. 9, 2022 -
Dexcom’s G7 glucose monitor wins awaited FDA clearance
The new Dexcom monitor is expected to generate significant patient demand, as it has been cleared by the FDA for patients with Type 1 and Type 2 diabetes, as well as gestational diabetes.
By Elise Reuter • Dec. 8, 2022 -
Edwards CEO Mussallem to retire, Bernard Zovighian named successor
Michael Mussallem will step down in May after leading the company for more than 20 years.
By Elise Reuter • Dec. 8, 2022 -
AdvaMed asks FDA to withdraw divisive draft guidance on LASIK surgery
Some comments were more supportive, with the American Optometric Association calling the agency’s proposal “timely and beneficial.”
By Nick Paul Taylor • Dec. 8, 2022 -
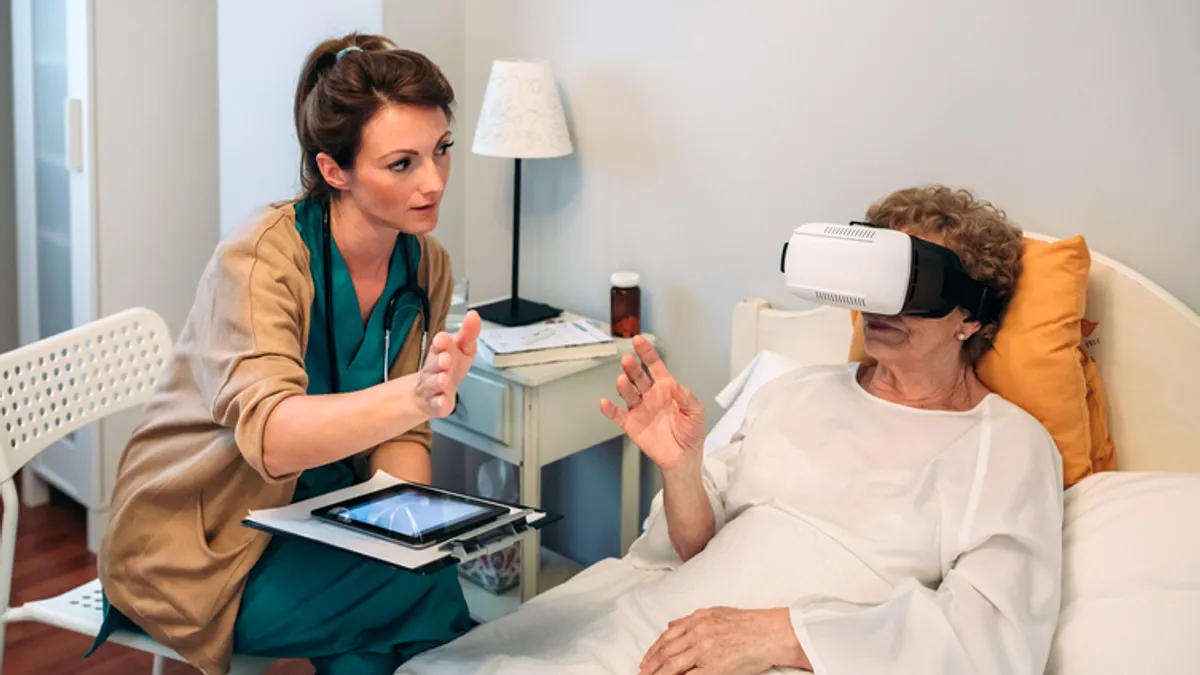
FDA outlines benefits and risks of emerging augmented and virtual reality medical devices
AR/VR may increase access to healthcare and make procedures less invasive, but could potentially cause cybersickness and head and neck strain.
By Nick Paul Taylor • Dec. 8, 2022 -
Apple prevails in AliveCor patent dispute ahead of potential Watch import ban
Patent board ruled that all 30 claims are unpatentable because they are obvious in view of other patents.
By Nick Paul Taylor • Dec. 8, 2022 -
Stanford’s smart bandages accelerate wound healing in preclinical tests
The bandages deliver electrical stimulation to accelerate wound healing in mice by 25% compared to standard sterile dressings.
By Nick Paul Taylor • Dec. 7, 2022 -
Ekso Bionics buys Indego lower limb exoskeleton business from Parker Hannifin for $10M
Indego and planned robotic assisted orthotic and prosthetic devices will slot into a portfolio of wearable robotics designed to tackle mobility loss.
By Nick Paul Taylor • Dec. 7, 2022 -
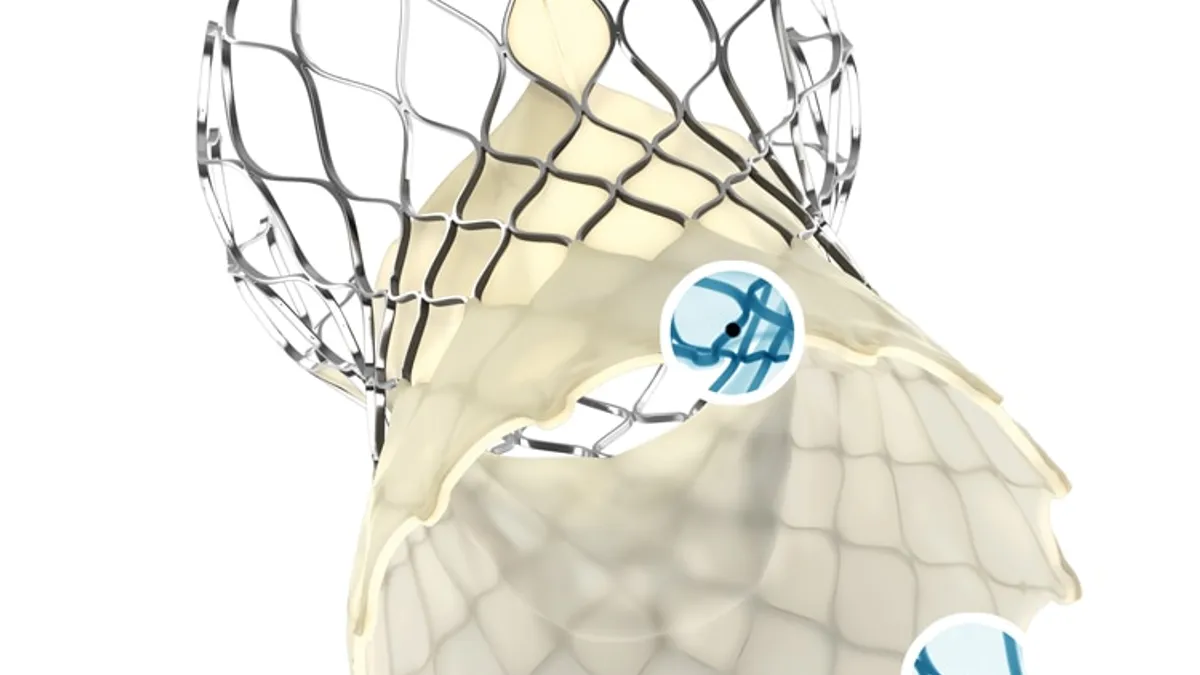
 Retrieved from Medtronic on September 19, 2022
Retrieved from Medtronic on September 19, 2022
Excess deaths to continue constraining recovery of TAVR market in 2023: Goldman
Hospital staffing shortages and excess deaths among seniors are expected to slow the recovery of structural heart procedures, Goldman Sachs analysts said.
By Nick Paul Taylor • Dec. 7, 2022 -
Abiomed’s Impella RP heart pump finds use limited after FDA review of critically ill patients
It's a setback for the company, which hoped the right ventricle pump would have a wider use. A post-approval study showed a low survival rate for the sickest patients.
By Elise Reuter • Updated Dec. 6, 2022 -
Zimmer, Boston Scientific set to lead positive medical device pricing trend in 2023: analysts
As inflation rose, medical device companies were able to secure higher prices in large contracts, which Goldman Sachs analysts wrote “should only be the beginning.”
By Nick Paul Taylor • Dec. 6, 2022