Medical Devices: Page 60
-
Abbott sees ‘signs of stability’ for 2023 as hospital staffing, supply chain improves
While the company continues to grapple with supply shortages and procedure restrictions, it sold more COVID-19 tests than expected in the fourth quarter.
By Elise Reuter • Jan. 25, 2023 -
Cybersecurity ‘more critical than ever’ in era of connected care: BD
The device maker’s annual report on the state of healthcare cybersecurity calls for better transparency and coordination across the industry.
By Susan Kelly • Jan. 25, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
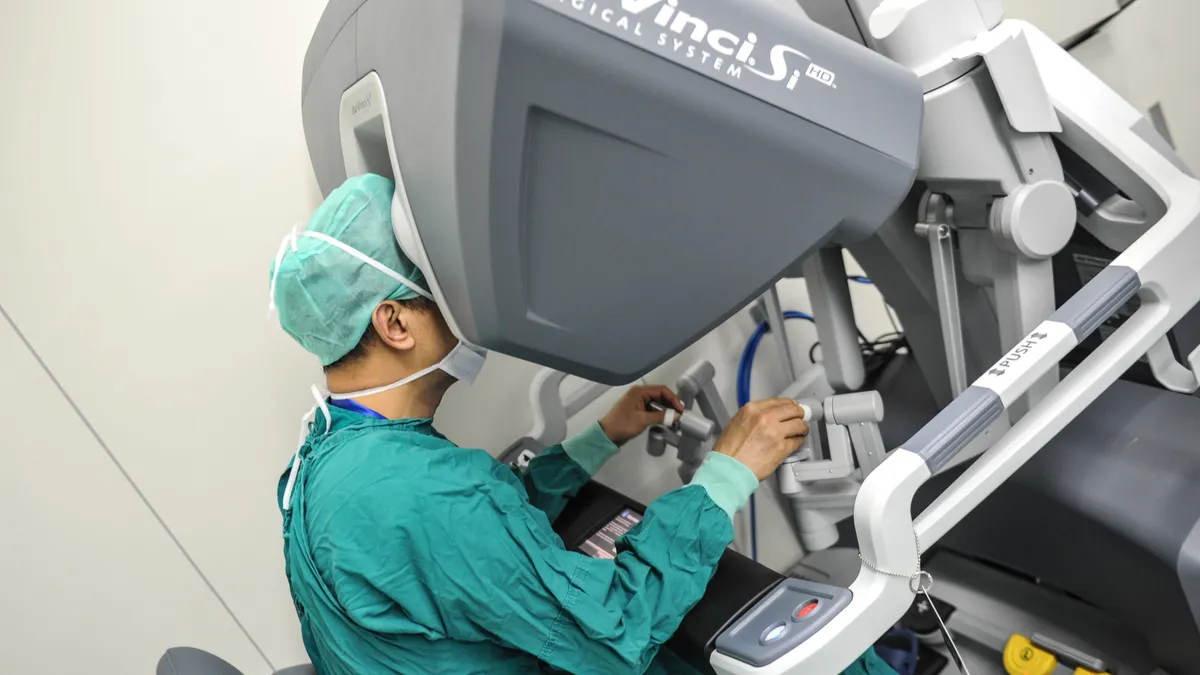
Intuitive Q4 profit slips on costs to boost share of robotic surgery market
The investment in innovation comes as Intuitive tries to maintain its dominant position in the face of rivals including Johnson & Johnson and Medtronic.
By Nick Paul Taylor • Jan. 25, 2023 -
3M’s healthcare unit Q4 earnings drop on nurse shortages, falling hospital budgets
Elective healthcare procedure volumes were about 90% of pre-COVID levels.
By Nick Paul Taylor • Jan. 25, 2023 -
J&J sees Abiomed adding to revenue in 2024; Q4 sales drop
Foreign-exchange fluctuations helped curb revenue in the period.
By Peter Green • Jan. 24, 2023 -
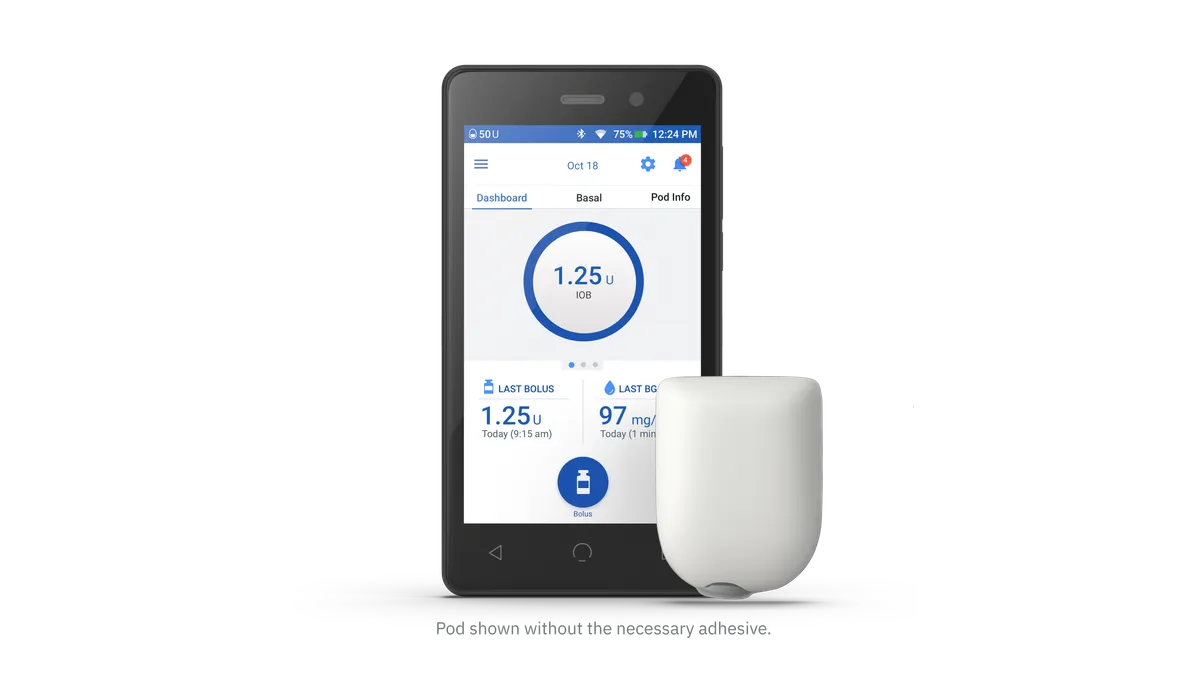
Insulet reports personal-data leak of 29,000 insulin pump customers
In a recall communication to users, Insulet shared information with its website performance and marketing partners through cookies and other trackers embedded in its website.
By Nick Paul Taylor • Jan. 24, 2023 -

 Courtesy of https://news.medtronic.com/Left-Ventricular-Assist-Device-For-Advanced-Heart-Failure#assets_34137_10-122:19299
Courtesy of https://news.medtronic.com/Left-Ventricular-Assist-Device-For-Advanced-Heart-Failure#assets_34137_10-122:19299
Medtronic asks HVAD users to return batteries with welding defects
The recall is one of several related to battery issues after the heart pump was pulled from the market.
By Elise Reuter • Jan. 23, 2023 -
Q&A
3 questions for Michael Snyder, lead inventor of single-drop blood test
The head of a Stanford research team says the test differs from Theranos' defunct product because "ours works."
By Peter Green • Jan. 23, 2023 -
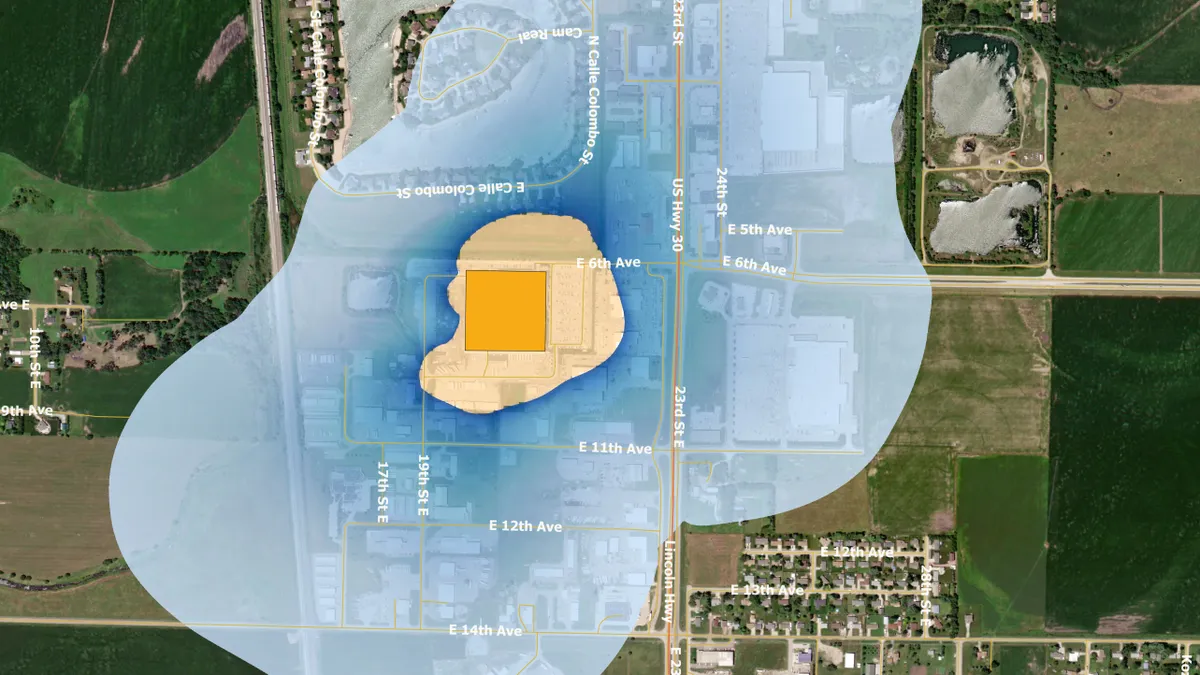
 EPA. (2022). "https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/forms/columbus-nebraska-becton-dickinson-pharmaceutical" [Photo]. Retrieved from EPA.gov.
EPA. (2022). "https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/forms/columbus-nebraska-becton-dickinson-pharmaceutical" [Photo]. Retrieved from EPA.gov.
Ethylene oxide regulation may disrupt device supply, AdvaMed warns White House
The trade group has asked President Joe Biden to consider the potential threat to patient care if facilities are shut down.
By Nick Paul Taylor • Jan. 23, 2023 -
FDA demands additional data on Abbott rival to Medtronic balloon, setting back approval timeline
Abbott’s partner Surmodics is “evaluating options” to reduce its spending while preparing a response to the regulator’s requests.
By Nick Paul Taylor • Jan. 20, 2023 -
Q&A
Dexcom CEO discusses 2023 plans, expanding to more patients with Type 2 diabetes
MedTech Dive caught up with Kevin Sayer for a conversation about forthcoming product launches and long-term plans to expand CGMs to a broader group of patients.
By Elise Reuter • Updated Jan. 20, 2023 -
Medtech hazards: Problems with Philips’ recall put safety of home-use devices at top of danger list
Patient safety group ECRI is challenging manufacturers to provide easy-to-follow device registration instructions and write simply worded recall notices.
By Nick Paul Taylor • Jan. 19, 2023 -
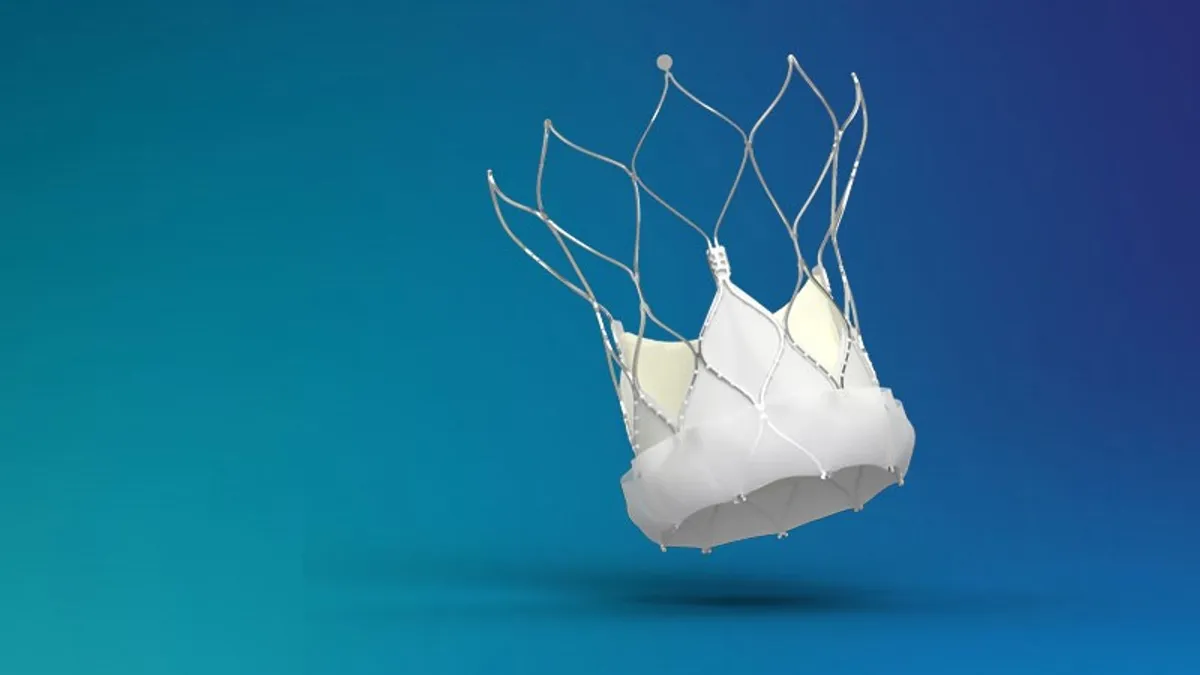
Abbott’s FDA approval for TAVI device tees up challenge to Edwards, Medtronic: analysts
Abbott has captured about 9% of the European market and aims to become a “credible third player” in the U.S.
By Nick Paul Taylor • Jan. 18, 2023 -
Shockwave to pay $100M for Neovasc, adding refractory angina treatment to device pipeline
Neovasc already has a CE mark for the device and last year said it was targeting a 2025 approval by the FDA.
By Nick Paul Taylor • Jan. 18, 2023 -
Medtech companies start 2023 on ‘optimistic’ note amid earnings forecasts, staffing: J.P. Morgan
Executive comments at the healthcare conference in San Francisco suggest companies expect staffing pressures and supply constraints to improve in 2023, analysts say.
By Elise Reuter • Jan. 17, 2023 -
AI predicts lung cancer risk six years into the future using single CT scan: study
A research team has made the deep learning model publicly available and says it has no intention to commercialize it.
By Nick Paul Taylor • Jan. 17, 2023 -
iRhythm shows signs of progress even as it ‘still has a way to go’ on key challenges: William Blair
The company should come close to its revenue estimate when reporting fourth quarter results, according to analysts.
By Nick Paul Taylor • Jan. 13, 2023 -
‘Mixed signals’ from medtech management suggest industry had bumpy end to 2022: analysts
The recovery of procedure volumes was “muted and uneven” in the fourth quarter with growth throttled by hospital staffing issues and worker absenteeism.
By Nick Paul Taylor • Jan. 13, 2023 -
Olympus gets two FDA warning letters over safety of reprocessed endoscopes
The regulator said the companies didn’t adequately test the device assembly process and waited too long to report problems from complaints.
By Elise Reuter • Jan. 12, 2023 -

 Courtesy of Press release https://verily.com/blog/verily-expands-stephen-gillett-s-role-to-president-and-chief-operating-officer/
Courtesy of Press release https://verily.com/blog/verily-expands-stephen-gillett-s-role-to-president-and-chief-operating-officer/
Google sibling Verily to lay off about 200 workers as it focuses on precision risk business
The company, which has more than 1,600 employees, will stop work on some products including its healthcare analytics software program.
By Nick Paul Taylor • Jan. 12, 2023 -
Intuitive forecasts 12%-16% growth in procedures in 2023 even as da Vinci robot sales slowed in Q4
For hospitals that have been reporting growth in robotic procedures, “da Vinci is a relative priority in their capital budgets,” the company said.
By Elise Reuter • Jan. 11, 2023 -
GE HealthCare to buy France’s IMACTIS in first acquisition as independent firm
IMACTIS’s CT-Navigation system is used to help surgeons in minimally invasive operations and reduce procedure time.
By Peter Green • Jan. 11, 2023 -
Rethinking 510(k): Studies show risk of using recalled devices as predicates for FDA clearance
Two studies found devices cleared through the FDA’s 510(k) pathway that listed recalled predicate devices were more likely to face recalls themselves.
By Nick Paul Taylor • Jan. 11, 2023 -
Sterigenics reaches $408M ethylene oxide settlement to resolve hundreds of lawsuits in Illinois
Sterigenics said “years of biased media coverage in the greater Chicago area” underpinned its decision to settle.
By Nick Paul Taylor • Jan. 11, 2023 -
How Abbott plans to make its Freestyle Libre a $10B product
CEO Robert Ford discussed plans to grow the market for Abbott’s continuous glucose monitors, and its broader diabetes strategy.
By Elise Reuter • Jan. 10, 2023