Medical Devices: Page 46
-
FDA improves 510(k) turnaround times, but PMA waits hit record high: analysis
The time it took for the FDA to reach 510(k) decisions decreased by 24 days, but the agency is still working through a pandemic-driven backlog of premarket approval applications.
By Nick Paul Taylor • July 24, 2023 -
BD gains FDA clearance to relaunch Alaris infusion pump after recalls
The company will fix or replace older devices still in use at hospitals as it ramps up to resume sales in its market-leading infusion business.
By Susan Kelly • July 24, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
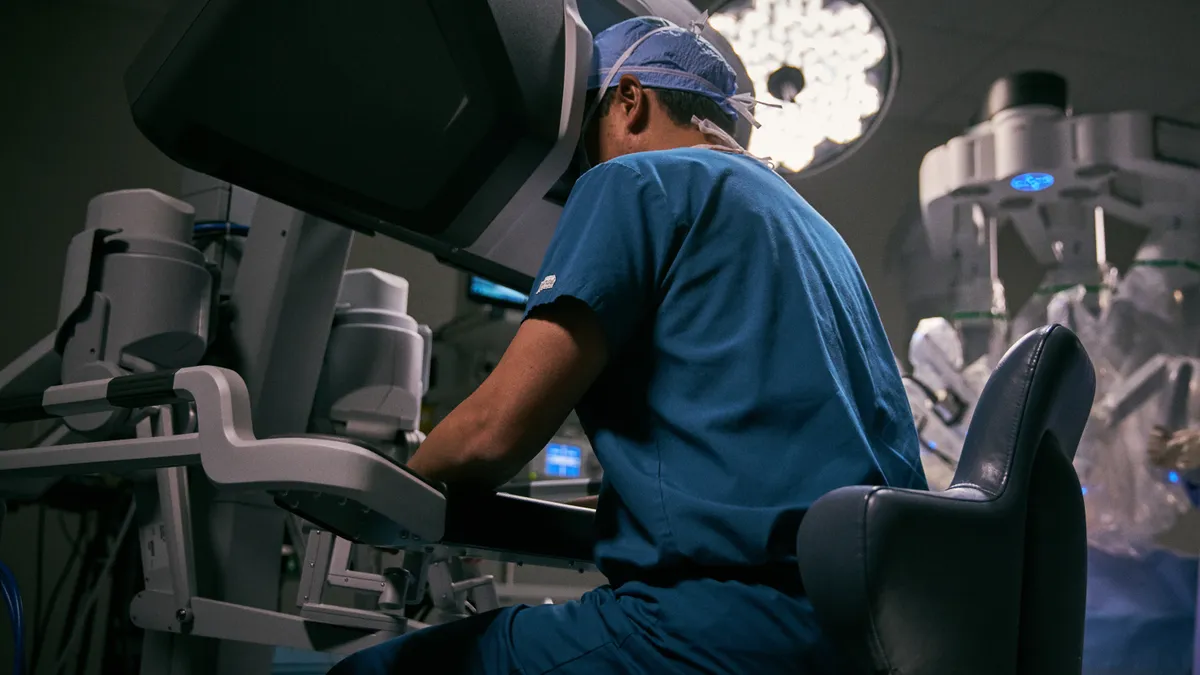
Bariatric surgery slowdown nips Intuitive Surgical’s Q2 procedure growth
New weight-loss drugs are eroding demand for surgical procedures that employ the company’s robotic systems.
By Susan Kelly • July 21, 2023 -
Opinion
A novel approach to IV access
Improvements in IV catheters can lead to better, more compassionate care, says Joseph Smith, BD’s chief scientific officer.
By Joseph Smith • July 20, 2023 -
Abbott Q2 net profit falls as weaker COVID test sales drag on revenue
Stronger medical device sales and a recovery in the infant formula business partly offset the impact of reduced COVID-19 testing.
By Susan Kelly • July 20, 2023 -
J&J earnings a ‘positive early indicator’ for medtech in Q2: analyst
The company raised its revenue and earnings forecast for the year, driven by new products and global procedure growth.
By Elise Reuter • July 20, 2023 -
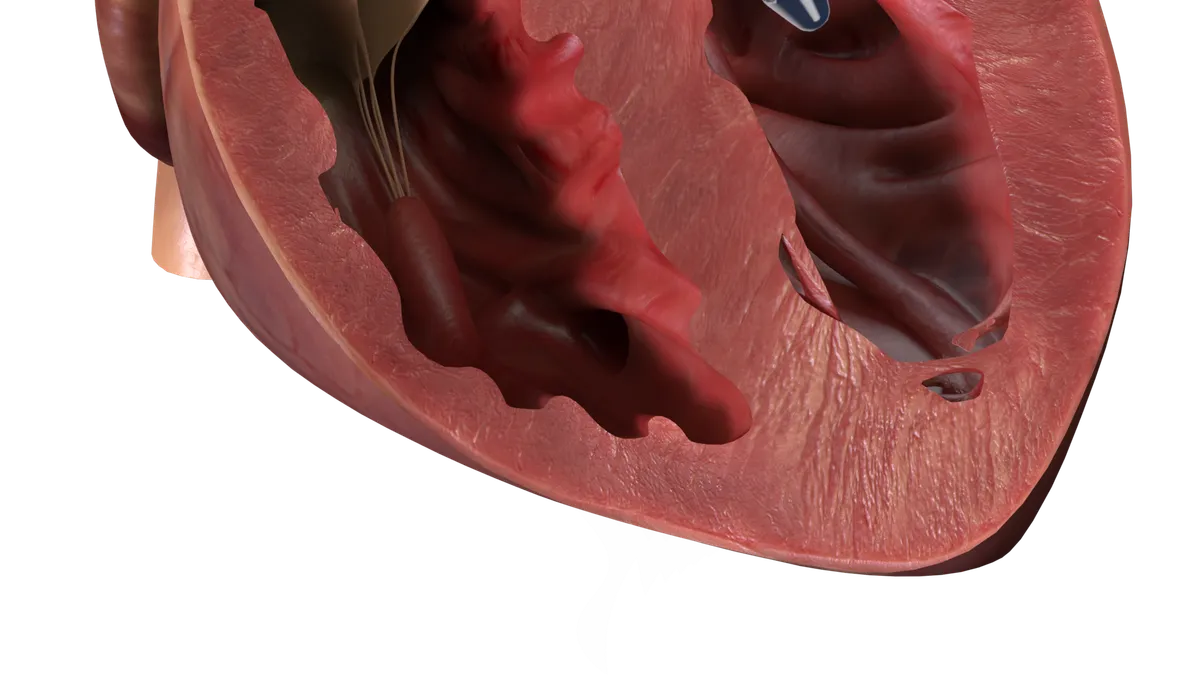
Four deaths attributed to interference between Abiomed’s heart pump and TAVR stents
There’s a risk that the valve implants can interact with the company’s Impella heart pumps, breaking the motor.
By Elise Reuter • Updated July 28, 2023 -
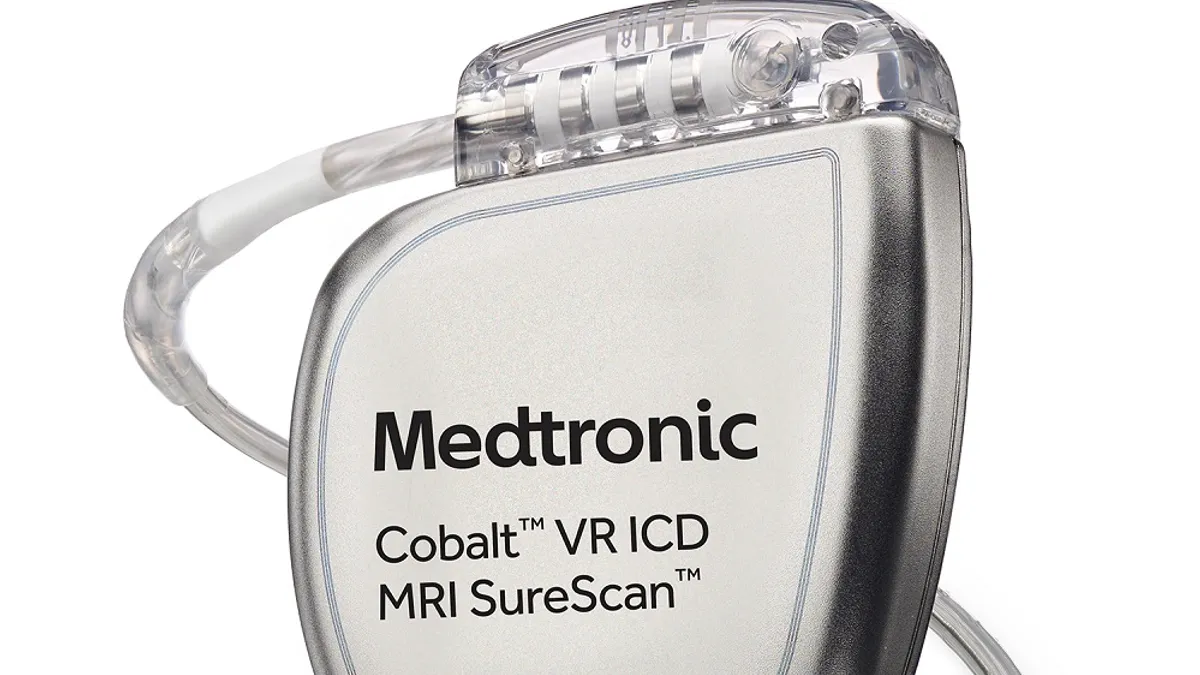
Medtronic recalls nearly 350,000 defibrillators for risk of reduced shock
The implantable devices may fail to deliver an electric shock to restore normal heart rhythm when needed, which could lead to a serious injury or death, the FDA said.
By Susan Kelly • July 18, 2023 -
Masimo plans cost cuts as Q2 revenue falls short of expectations
Shares of the patient-monitoring device maker dropped 22% after it said hospital orders for its equipment were delayed and demand for consumer products softened.
By Susan Kelly • July 18, 2023 -
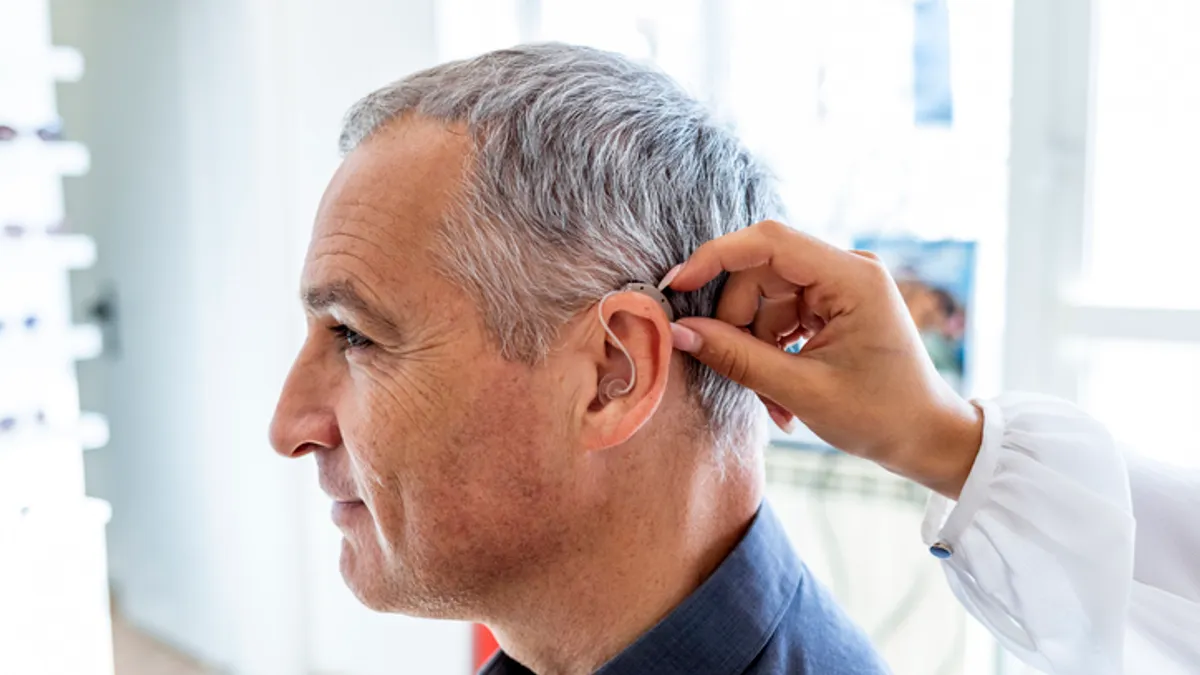
Hearing aids fail to improve cognition in randomized trial, but subgroup analysis offers hope
While there were no positive effects observed in healthy older adults, a smaller analysis pointed to a difference in those at higher risk of cognitive decline.
By Nick Paul Taylor • July 18, 2023 -
Big Health buys Limbix to add adolescent depression app to digital therapeutic portfolio
The phone-based treatment, SparkRx, uses self-guided, cognitive behavioral therapy to teach skills such as mood tracking.
By Nick Paul Taylor • July 18, 2023 -
ViewRay files for Chapter 11 bankruptcy
The company, which makes an MRI-guided radiation therapy system, intends to sell all or some of its assets.
By Elise Reuter • July 17, 2023 -
FDA provides safety update on hernia repair mesh as medtech companies face lawsuits
The FDA’s analysis of adverse event reports describes problems including pain, injury and disability.
By Nick Paul Taylor • July 17, 2023 -
AdvaMed says CMS radiotherapy rate cut proposal will limit patient access
The proposed rule includes a 2% reduction in reimbursement, continuing a trend that has seen the CMS cut payments by more than 20% over the past decade, according to the the American Society for Radiation Oncology.
By Nick Paul Taylor • July 17, 2023 -
What the new CMS payment proposals mean for device makers
Ophthalmic device company Glaukos could benefit from the proposed changes, analysts wrote.
By Elise Reuter • July 14, 2023 -
Problem with Draeger transport ventilators categorized as Class I recall by FDA
Draeger contacted customers last month after receiving reports that devices stopped providing ventilation because of depleted batteries.
By Nick Paul Taylor • July 14, 2023 -
Court allows Axonics neurostim patent case to proceed against Medtronic
A federal appeals court found that a U.S. Patent Office board erred in its legal analysis of the challenge to Medtronic’s neurostimulation patents.
By Susan Kelly • Updated July 17, 2023 -
Becton Dickinson flags cybersecurity vulnerabilities in Alaris system
The company identified eight security issues affecting its infusion pump system, including one high-risk concern.
By Elise Reuter • July 13, 2023 -
Urotronic wins FDA approval to challenge Boston Scientific, Teleflex for prostate market
The company’s Optilume device combines balloon dilation and the delivery of paclitaxel to treat symptoms linked to an enlarged prostate.
By Nick Paul Taylor • July 13, 2023 -
Illumina hit by EU with maximum €432M fine for Grail acquisition
The European Commission called Illumina’s closing of the deal without its approval an “unprecedented” move that undermines its system for regulating the competitive landscape.
By Susan Kelly • July 12, 2023 -
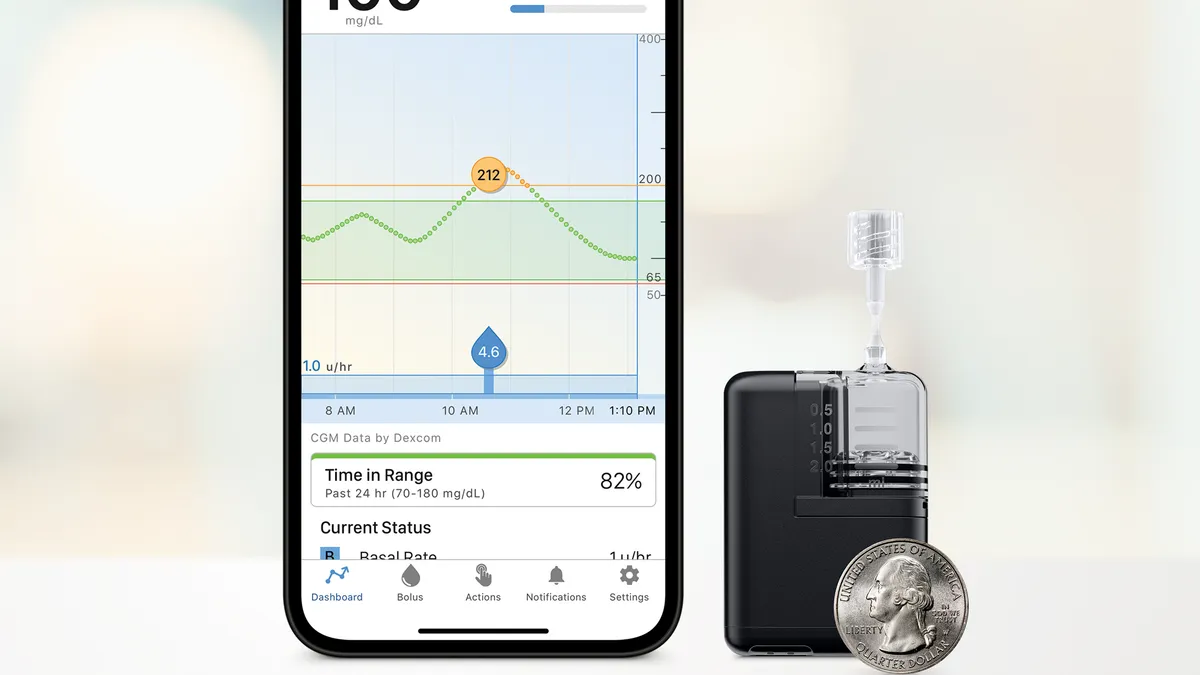
Tandem’s Mobi insulin pump could reaccelerate growth next year: analyst
The device, which is smaller than Tandem’s past pumps, could drive more people to switch from insulin injections, an analyst with Craig-Hallum said.
By Elise Reuter • July 12, 2023 -
Analysis shows paclitaxel-coated devices unlikely to increase death risk, leading FDA to withdraw warning
The FDA’s decision to remove mortality warnings from device labels is a boost to manufacturers of paclitaxel-coated devices.
By Nick Paul Taylor • July 12, 2023 -
J&J Megadyne unit’s electrode pad recall labeled Class I over burn risk
The problem, which can cause third-degree burns, has been linked to 63 injuries and no deaths.
By Nick Paul Taylor • July 12, 2023 -
Fresenius Medical Care names Martin Fischer as CFO
Fischer joins the company after former CFO Helen Giza was promoted to CEO.
By Elise Reuter • July 11, 2023 -
Analysts expect a reimbursement boost for Shockwave coronary artery-clearing device
The analysts think growth expectations for the company are too low and have set their earnings estimate for next year 15% above Wall Street’s consensus.
By Nick Paul Taylor • July 11, 2023