Medical Devices: Page 4
-
Trump tariffs
How Trump’s trade fight could impact the medtech industry
Relocating manufacturing would require substantial capital investment, but the timespan for the president’s new tariffs is unclear, supply chain experts said.
By Susan Kelly • Feb. 12, 2025 -
EMA creates advice portal for some high-risk medical devices
The European Medicines Agency said the portal will enable consultations with medical device expert panels that can promote faster access to new technologies.
By Nick Paul Taylor • Feb. 12, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Inspire Medical reveals DOJ civil investigation
CEO Tim Herbert told investors the company received a civil investigative demand from the Department of Justice in January for information on marketing and reimbursement practices.
By Ricky Zipp • Feb. 11, 2025 -
Mass General Brigham to conduct largest layoffs in its history
MGB said the layoffs were necessary to get ahead of an anticipated budget shortfall of $250 million over the next two years.
By Susanna Vogel • Feb. 11, 2025 -
Former FDA head Gottlieb calls for less regulation of certain AI tools
Gottlieb, who led the agency during the first Trump presidency, supports a reversion to an earlier interpretation of the 21st Century Cures Act.
By Elise Reuter • Feb. 11, 2025 -
M&A and a separation take center stage in latest earnings season
BD announced it would split from its biosciences and diagnostics unit, while Zimmer Biomet updated investors on its $1.1 billion buy of Paragon 28.
By Susan Kelly • Feb. 10, 2025 -
FDA posts early alert about BD device linked to 30 injuries, 4 deaths
The alert was posted one day after BD sent customers a letter warning about the risk of atherectomy catheters breaking.
By Nick Paul Taylor • Feb. 10, 2025 -
Thermo Fisher lays off another 300 workers in Massachusetts
Job cuts in Cambridge and Plainville are the second workforce reduction planned for the facilities in the past three months.
By Susan Kelly • Feb. 7, 2025 -
BD plans split from life science business to fuel medtech investment
CEO Tom Polen said BD will “double down on shifting our portfolio, both organically and inorganically through tuck-in M&A, into higher-growth, high-margin accretive growth spaces.”
By Nick Paul Taylor • Feb. 7, 2025 -
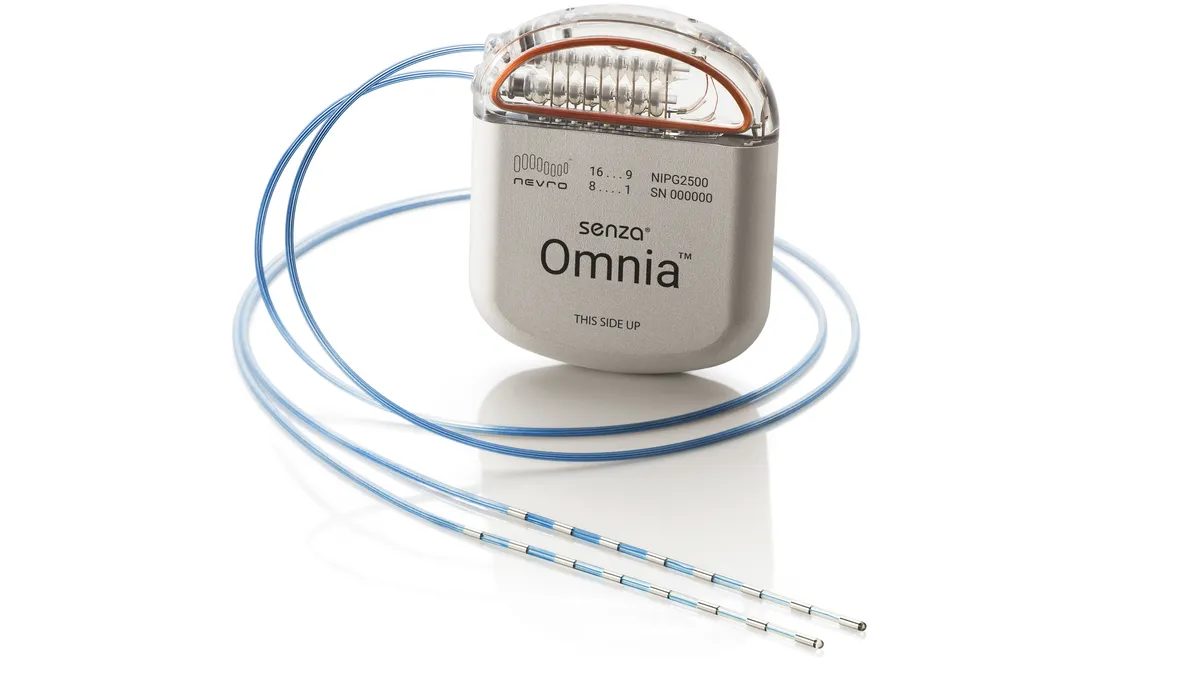
Globus to buy Nevro for $250M
Analysts said Nevro’s valuation was “inexpensive,” but the spinal cord stimulation business can benefit from Globus’ scale and orthopedic relationships.
By Elise Reuter • Feb. 6, 2025 -
Zimmer touts ASC opportunity with Paragon 28 purchase
With the $1.1 billion acquisition, Zimmer Biomet expects its sports medicine, extremities and trauma segment will outpace hips and knees.
By Elise Reuter • Feb. 6, 2025 -
Q&A
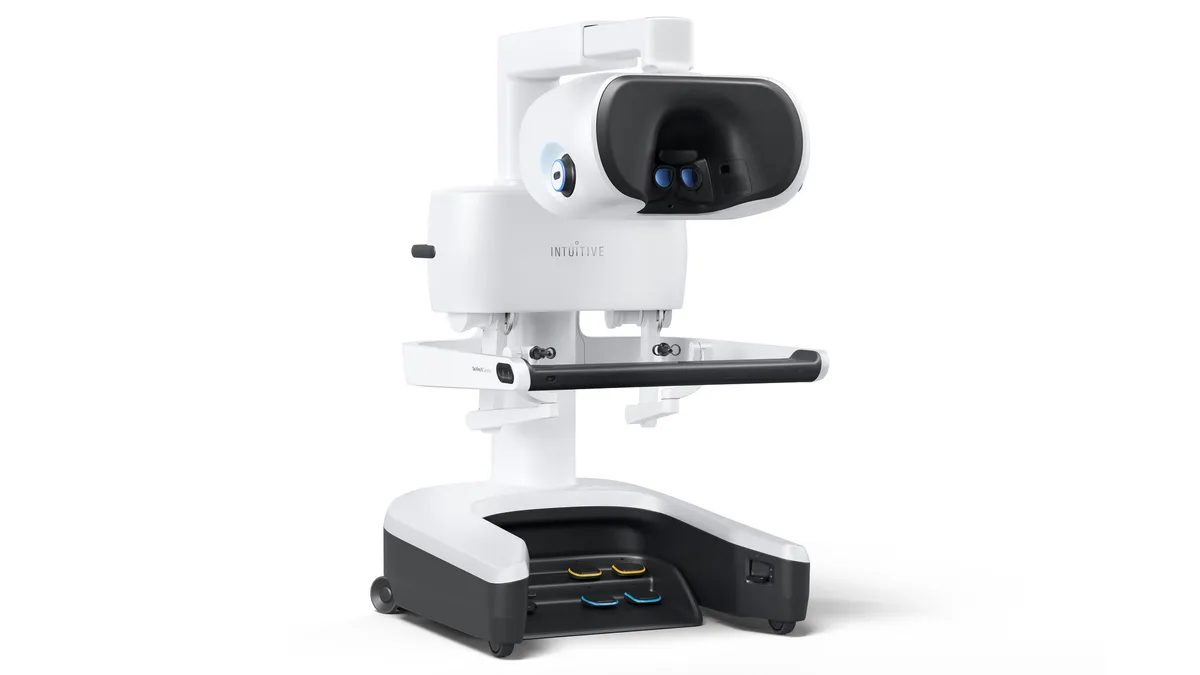
Medtronic’s Orleigh Bogle on changing the culture of surgical training
Bogle, Medtronic’s head of medical affairs for digital surgery, also discussed the company’s plans to train surgeons for its Hugo surgical robot.
By Elise Reuter • Feb. 6, 2025 -
Trump tariffs
AHA joins call for Trump to exempt devices from import tariffs
Disruption to the supply of devices from China would curtail hospitals' ability to perform life-saving surgeries and protect patients and healthcare workers from contagion, the group warned.
By Nick Paul Taylor • Feb. 6, 2025 -
Pulse Biosciences names new CFO, continuing string of C-suite changes
Pulse, which named a new CEO last month, is pitching its pulsed field ablation technology as a way to shorten procedure times.
By Nick Paul Taylor • Feb. 5, 2025 -
Boston Scientific closes its year of PFA on a high note
Farapulse, Boston Scientific’s pulsed field ablation product, fueled year-over-year growth of nearly 171% in the fourth quarter for the company’s electrophysiology group.
By Ricky Zipp • Feb. 5, 2025 -
Trump tariffs
Illumina placed on China’s unreliable entity list
Analysts said the move comes amid rising trade tensions with the U.S. and as Illumina faces stiff competition in China’s genome sequencing market.
By Susan Kelly • Feb. 5, 2025 -
Medtronic recall of brain fluid drainage systems linked to 15 injuries
The company will keep the systems on the market but is asking providers to check devices for visible cracks and return affected devices.
By Nick Paul Taylor • Feb. 4, 2025 -
RFK Jr. clears key hurdle on path to HHS secretary
A key Senate committee voted to advance Robert F. Kennedy’s nomination even after some legislators raised concerns about his previous vaccine stances and confusion surrounding programs like Medicare and Medicaid.
By Sydney Halleman , Delilah Alvarado , Jonathan Gardner • Feb. 4, 2025 -

 Retrieved from Olympus on February 03, 2025
Retrieved from Olympus on February 03, 2025
FDA labels recall of discontinued Olympus endoscope part as Class I
The update comes about six weeks after an early alert flagged reports of 120 injuries and one death linked to reprocessing of the device.
By Susan Kelly • Feb. 3, 2025 -
Trump tariffs
Advamed pushes for medical device exemption from Trump tariffs
CEO Scott Whitaker said “an exemption was provided for most medical devices during President Trump’s first term with respect to the tariffs on China, and we are advocating for a similar approach.”
By Susan Kelly • Feb. 3, 2025 -
Baxter CEO José Almeida retires
Brent Shafer, Baxter’s lead independent director, will serve as interim CEO. The company also promoted Heather Knight to chief operating officer.
By Elise Reuter • Feb. 3, 2025 -
Trump issues tariffs on Canada, Mexico and China
The president ordered an additional 10% duty on goods from China and 25% tariffs on imports from Canada and Mexico, though Canadian energy resources will face a lower rate.
By Philip Neuffer • Updated Feb. 1, 2025 -
Troy Tazbaz, CDRH digital health leader, resigns
Tazbaz was director of the FDA’s Digital Health Center of Excellence for two years, leading key policies on artificial intelligence and software as a medical device.
By Elise Reuter • Jan. 31, 2025 -
2025 outlook
Top medtech trends in 2025: New products and M&A are bright spots, but Trump brings uncertainty
Experts highlighted surgical robotics, artificial intelligence and M&A as top trends this year. However, the Trump administration could create challenges for the industry.
By Ricky Zipp • Jan. 31, 2025 -

 Retrieved from Nasdaq on January 31, 2025
Retrieved from Nasdaq on January 31, 2025
Beta Bionics raises $204M in IPO
The diabetes tech firm began trading this week and was valued at $966.2 million as of Thursday.
By Elise Reuter • Jan. 31, 2025