Medical Devices: Page 37
-
Acutus laying off 65% of employees to focus solely on Medtronic heart device deal
The company has decided to exit the mapping and ablation business and focus its remaining cash on the Medtronic partnership.
By Nick Paul Taylor • Nov. 10, 2023 -
4 takeaways from Stryker’s investor day
The company’s expected return to pre-pandemic operating margins, procedure backlogs and weight loss drugs were among the topics discussed at Wednesday’s event.
By Elise Reuter • Nov. 9, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
BD’s 2024 forecast comes in ‘well below’ Wall Street’s expectations
Wall Street analysts called out BD’s disappointing 2024 EPS forecast in notes to investors, following a Thursday morning earnings call.
By Ricky Zipp • Nov. 9, 2023 -
NICE backs use of hybrid closed loop systems for Type 1 diabetes, if companies offer discounts
The group’s recommendation gives around 150,000 people with Type 1 diabetes living in England the chance to access the systems, according to an NHS England official.
By Nick Paul Taylor • Nov. 9, 2023 -
J&J’s Ottava robot arriving too late to threaten Intuitive: analysts
With J&J years from approval and hospitals committed to the da Vinci system, analysts see little threat to Intuitive for the foreseeable future.
By Nick Paul Taylor • Nov. 9, 2023 -
Masimo lowers outlook, citing constrained hospital budgets
The patient monitoring company is also facing a slowdown in demand for the consumer audio products it acquired in a deal last year.
By Susan Kelly • Nov. 8, 2023 -
Recor wins FDA approval for renal denervation system
The approval makes Recor the first company to bring the treatment to the U.S. after getting an FDA panel nod in August.
By Elise Reuter • Nov. 8, 2023 -
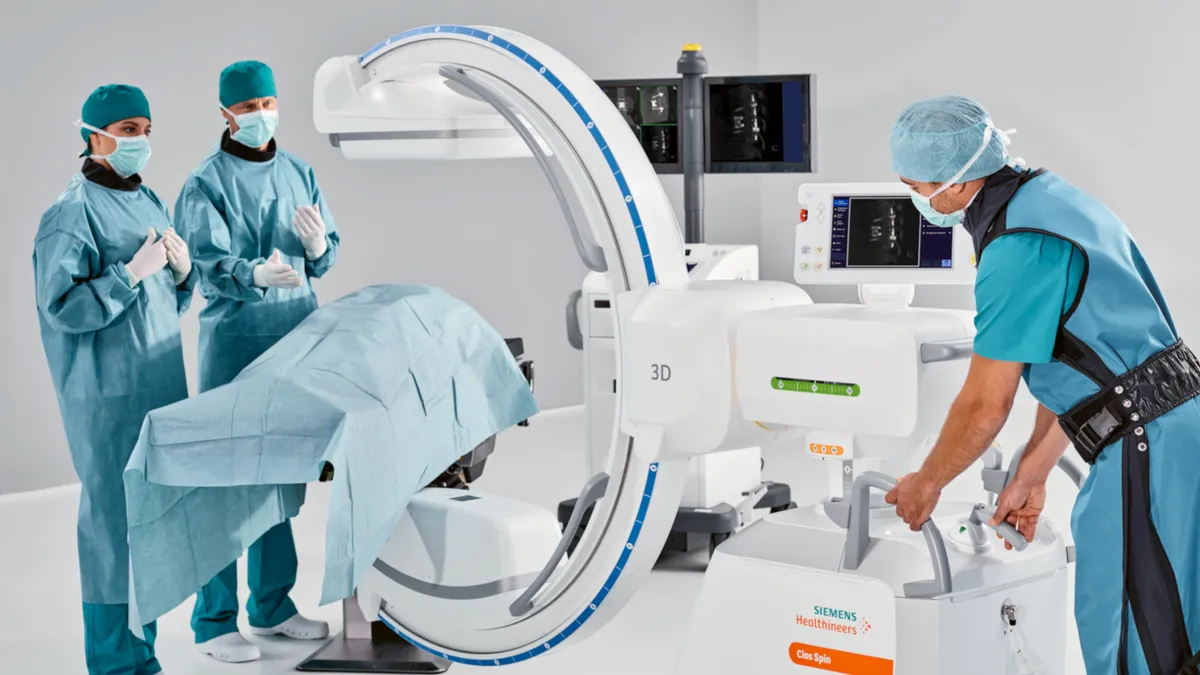
 Retrieved from Siemsens Website on July 01, 2022
Retrieved from Siemsens Website on July 01, 2022
Siemens Healthineers’ Varian rebounds; execs mum on diagnostics sale rumors
The company's diagnostics unit continued to struggle amid layoffs and reports of a potential sale.
By Nick Paul Taylor • Nov. 8, 2023 -
Intuitive will weather weight-loss drug pressures, could see sales boost in ‘24: William Blair
Obesity drugs are constraining Intuitive's growth today, but the robotic surgery specialist could receive a sales boost in 2024 as patients reschedule bariatric procedures.
By Nick Paul Taylor • Nov. 8, 2023 -
J&J targets 2024 for Ottava FDA filing
The company expects to file a submission for an investigational device exemption in the second half of 2024 that will allow it to begin clinical trials for the robotic surgery system.
By Susan Kelly • Nov. 7, 2023 -
Zimmer Biomet’s Ivan Tornos sets priorities for first year as CEO
Tornos, who took the helm in August, said the company will grow through internal innovation and M&A.
By Elise Reuter • Nov. 7, 2023 -
25 attorneys general urge FDA to act fast to mitigate risk of racial bias in pulse oximetry
The AGs want the FDA to “require manufacturers and vendors of pulse oximeters to include clear, comprehensible and evidence-based warning labels.”
By Nick Paul Taylor • Nov. 7, 2023 -
Siemens Healthineers to lay off 300 workers, move some manufacturing to Ireland
The moves are part of a larger restructuring plan as its diagnostics business faces economic challenges.
By Ricky Zipp • Nov. 6, 2023 -
J&J links radiofrequency catheter to improved quality of life in atrial fibrillation study
Scores on a quality-of-life scale improved and use of antiarrhythmic drugs fell after people underwent treatment with the device.
By Nick Paul Taylor • Nov. 6, 2023 -
Sponsored by Shyft Global Services
The 6 Rs of sustainability and the Hippocratic Oath
Learn how key pillars of the Hippocratic Oath can guide sustainability for medical device OEMs.
Nov. 6, 2023 -
5 companies to watch as medtech earnings wind down
Zimmer Biomet, BD, Masimo, Illumina and Medtronic are all set to report financial results as the medtech earnings season draws to a close.
By Nick Paul Taylor • Nov. 6, 2023 -
Stryker in ‘sprint back to 2019’ as procedures grow and supply chain, staffing pressures improve
The company expects a procedure backlog to boost orthopedic surgery demand in the fourth quarter and through 2024.
By Ricky Zipp • Nov. 3, 2023 -
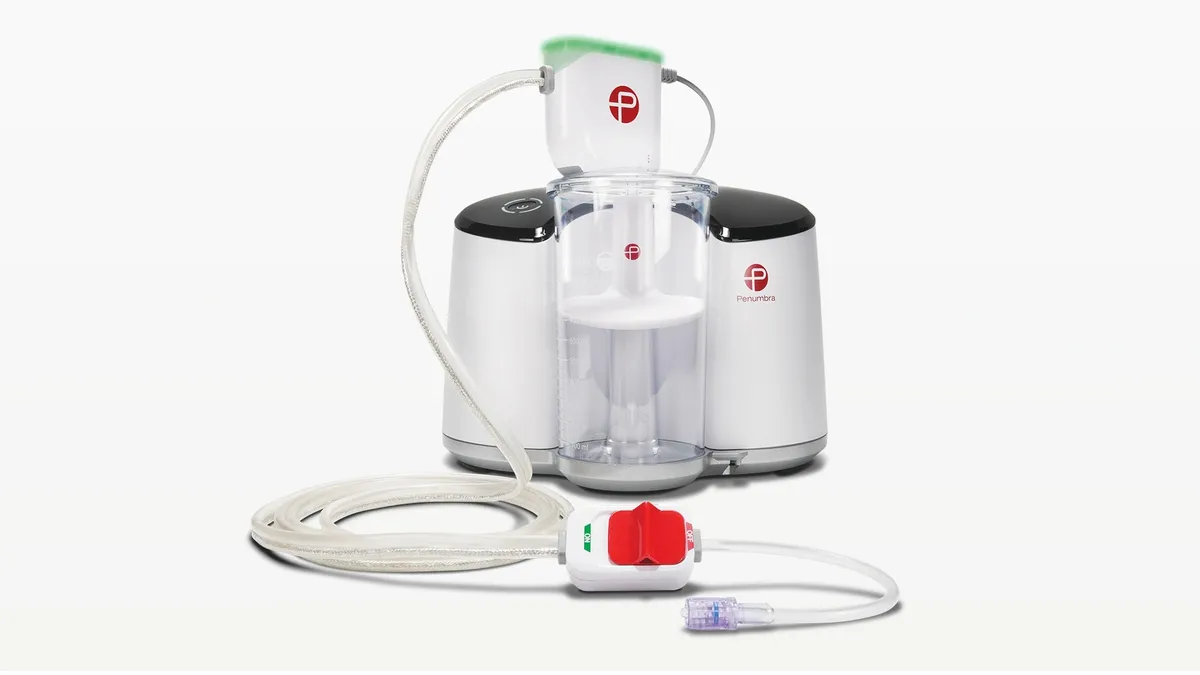
Penumbra touts benefits of vacuum thrombectomy for PE in late-breaking data
An interim analysis of Penumbra’s Indigo aspiration system met its primary safety and efficacy criteria, according to data shared at the Vascular Interventional Advances Conference.
By Elise Reuter • Nov. 3, 2023 -
 Retrieved from Google on May 28, 2020
Retrieved from Google on May 28, 2020
Elective procedure interest still topping pre-COVID levels, survey finds
Google searches for 20 elective surgeries in the U.S. were at 112% of pre-COVID levels in the week ended Oct. 28, according to a report from Needham analysts.
By Susan Kelly • Nov. 3, 2023 -
Inari Medical to buy limb-saving device maker LimFlow for up to $415M
The deal includes $250 million in cash upfront and additional payments based on reaching commercial and reimbursement milestones.
By Susan Kelly • Nov. 3, 2023 -
Baxter completes biopharma solutions sale, business unit revamp
The company plans to use most of the $3.7 billion in after-tax proceeds from the biopharma solutions sale to repay debt.
By Susan Kelly • Nov. 2, 2023 -
CooperCompanies buys reproductive health products from Cook Medical after failed takeover
The company paid $300 million to buy certain maternal fetal medicine and gynecological surgery products.
By Elise Reuter • Nov. 2, 2023 -
FDA extends pandemic-era policy allowing certain device, production changes without prior approval
Companies can make limited modifications on certain devices and manufacturing processes to address supply shortages or production limitations.
By Ricky Zipp • Nov. 2, 2023 -
Drilling down on obesity drugs: What medtech executives are saying
Investor concerns that interest in GLP-1s will slow demand for medical devices and procedures have shaved roughly $370 billion in value from stocks across the medtech sector, according to research by Mizuho.
By Susan Kelly • Nov. 2, 2023 -
White House orders HHS to start collecting reports on the safety of AI in healthcare
President Joe Biden outlined the planned program as part of an executive order on “safe, secure, and trustworthy” artificial intelligence.
By Nick Paul Taylor • Nov. 1, 2023