Medical Devices: Page 34
-
Apple to pause US sales of smartwatches with pulse oximetry
The tech giant’s move follows a USITC ruling that found the watches infringed some of Masimo's patents for measuring blood oxygen.
By Susan Kelly • Dec. 20, 2023 -
FDA drafts guidance outlining real-world evidence for medical device submissions
By issuing the new guidance, the agency is meeting the requirements of a year-end spending bill.
By Elise Reuter • Dec. 19, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Zimvie to sell spine business for $375M
Zimvie said the sale to H.I.G. Capital will leave the company focused on its dental business and help reduce debt.
By Elise Reuter • Dec. 18, 2023 -
BTIG analysts optimistic medtech will rebound in 2024 after ‘string of rough years’
Rising interest rates, fears about the impact of GLP-1 drugs and “choppy supply-chain dynamics” dragged on the industry this year, the analysts wrote.
By Nick Paul Taylor • Dec. 18, 2023 -
Glaukos wins FDA approval for drug-releasing eye implant to treat glaucoma
William Blair analysts said the product “should revolutionize how glaucoma is treated by addressing noncompliance with drops.”
By Nick Paul Taylor • Dec. 15, 2023 -
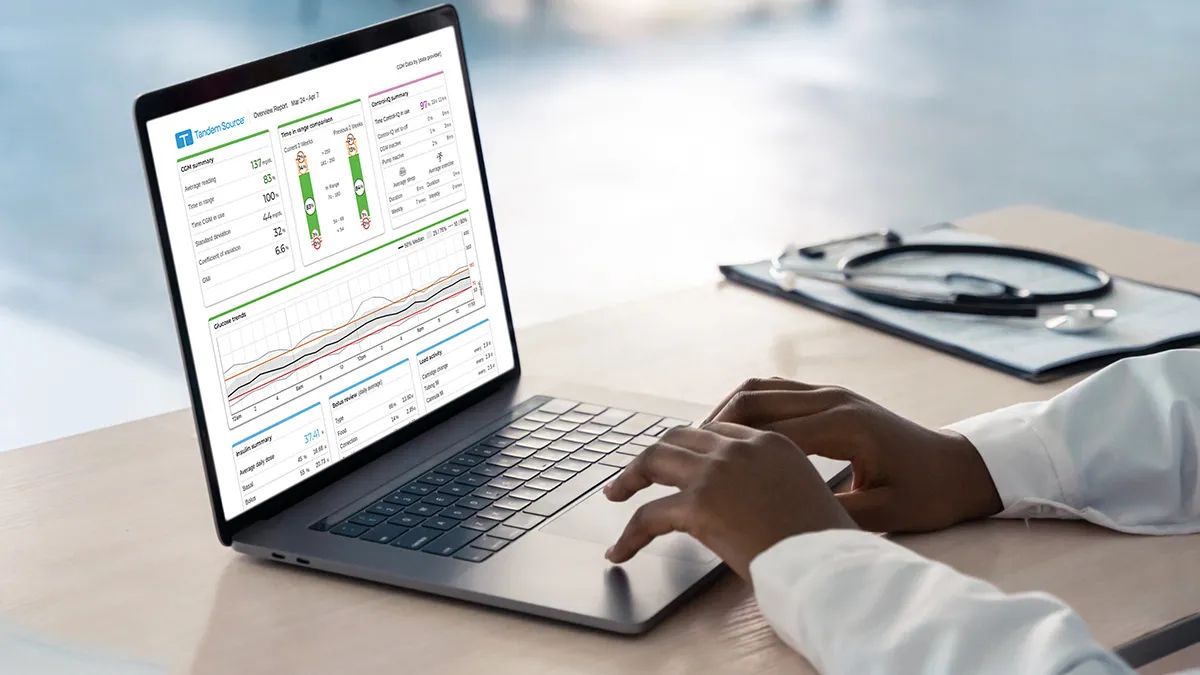
Tandem completes US launch of revamped diabetes management platform
The platform, Tandem Source, combines the features of the company’s legacy t:connect offerings with new data reports.
By Nick Paul Taylor • Dec. 15, 2023 -
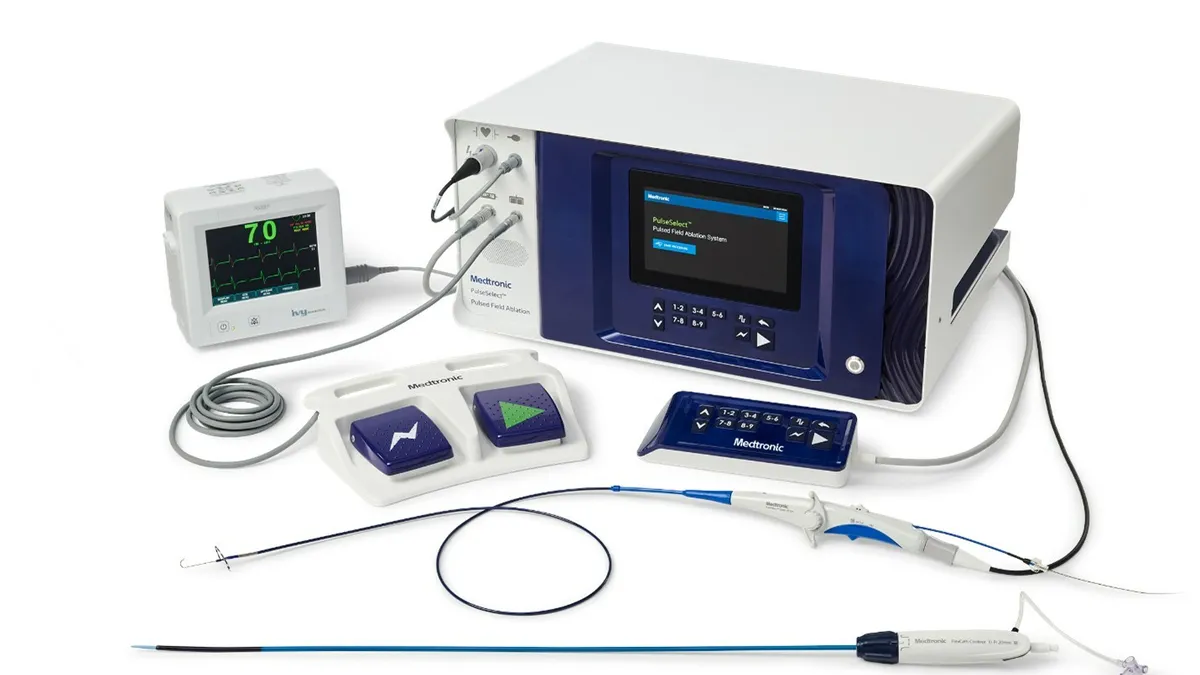
Medtronic snags first pulsed field ablation approval in US
The treatment for atrial fibrillation has gained attention as a safer alternative to radiofrequency and cryoablation. Boston Scientific and Johnson & Johnson are also pursuing the PFA market.
By Susan Kelly • Dec. 14, 2023 -
Resmed hails ‘significant victory’ as patent board rules in dispute with NYU
The decisions invalidate claims that NYU argued were infringed by Resmed’s sleep apnea devices.
By Nick Paul Taylor • Dec. 14, 2023 -
In wake of Philips recall, senators urge review of FDA medical device oversight
Sens. Richard Durbin and Richard Blumenthal said the sleep apnea device maker “did nothing” while patients suffered.
By Susan Kelly • Dec. 14, 2023 -
Integra to buy J&J’s Acclarent for $275M
Integra said the purchase, which includes a portfolio of balloon dilation products for the sinuses and eustachian tube, would make it a market leader in ENT procedures.
By Elise Reuter • Dec. 13, 2023 -
Zimvie’s collaboration with Brainlab delivers 510(k) win for spinal fixation system
Amid recent struggles in its spine unit, Zimvie has been expanding its Brainlab partnership.
By Nick Paul Taylor • Dec. 13, 2023 -
The FDA approved 2 renal denervation devices. There are still questions about who will benefit.
The devices from Recor and Medtronic are intended to treat high blood pressure. An advisory panel backed the former, but recommended against approval of the latter.
By Elise Reuter • Dec. 13, 2023 -
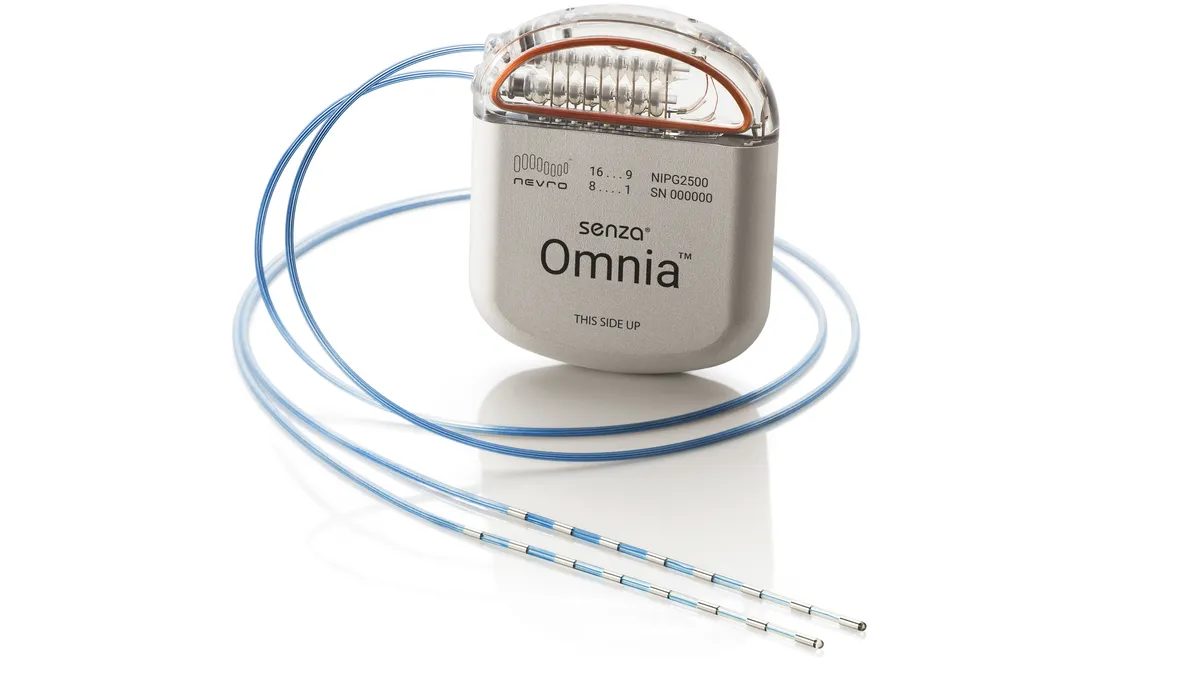
Activist investor takes stake in Nevro amid struggles to grow neuromodulation sales
Engaged Capital is opposed to Nevro going after acquisitions that may jeopardize its core business, Bloomberg reported Monday.
By Nick Paul Taylor • Dec. 12, 2023 -
Why heart device makers are investing in left atrial appendage closure
Medtronic and Johnson & Johnson are pursuing a market that leader Boston Scientific predicts could hit $6 billion by 2030.
By Susan Kelly • Dec. 12, 2023 -
Hologic promotes Essex Mitchell to COO
Mitchell joined Hologic as vice president of sales and commercial excellence for the GYN surgical division in 2017.
By Nick Paul Taylor • Dec. 11, 2023 -
Medtronic expands AI endoscopy partnership with Cosmo
The collaboration will focus on Cosmo Intelligent Medical Devices’ GI Genius module, which Medtronic distributes globally.
By Elise Reuter • Dec. 11, 2023 -
Advamed adds medical imaging division, names new board chair
The medtech association is bringing on staff from the Medical Imaging & Technology Alliance to lead the new division.
By Elise Reuter • Dec. 11, 2023 -
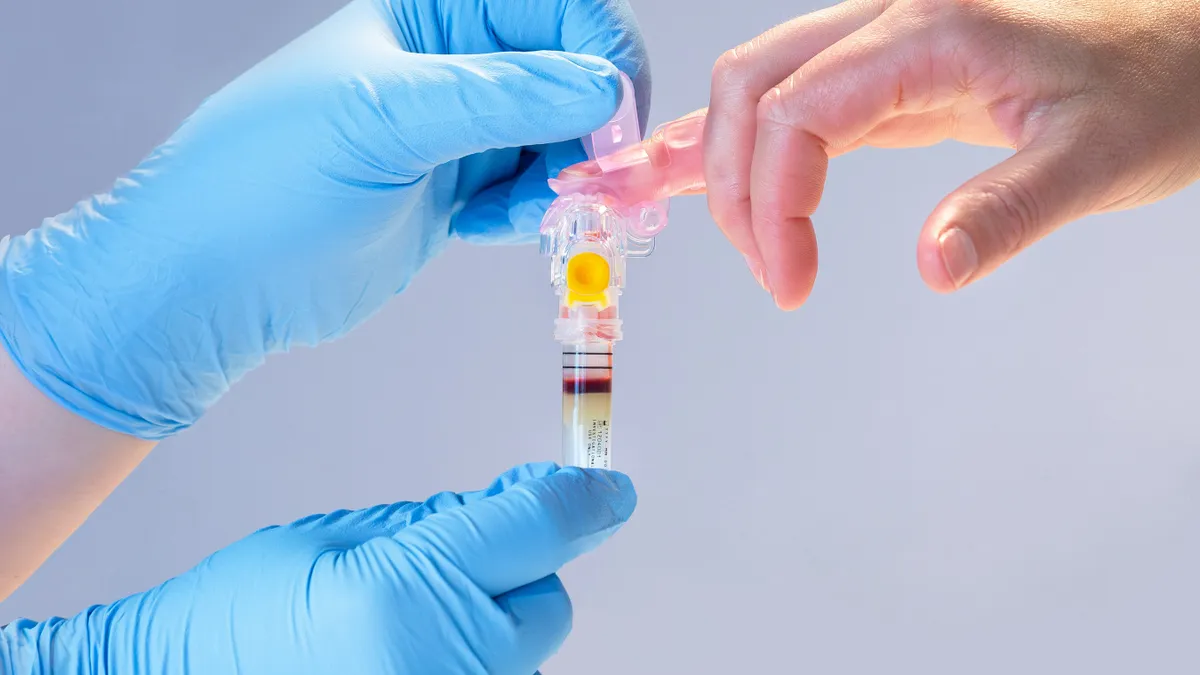
BD receives 510(k) clearance for fingerstick blood test sample collection device
The clearance positions BD and partner Babson Diagnostics to support blood collection from community sites such as pharmacies.
By Nick Paul Taylor • Dec. 8, 2023 -
Medtronic secures CE mark for rechargeable Percept deep brain stimulation device
The company plans to make the device available in Western Europe this month and “launch in additional regions based on local regulations.”
By Nick Paul Taylor • Updated Dec. 13, 2023 -
Medtronic scraps plans to buy insulin patch-pump maker EOFlow
Medtronic cited “multiple breaches” of the companies’ agreements. EOFlow has been embroiled in patent lawsuits with Insulet this year.
By Elise Reuter • Dec. 7, 2023 -
5 takeaways from J&J’s investor day
The company outlined its long-term growth expectations and confirmed that it has no plans to separate its medtech and pharma segments.
By Elise Reuter • Dec. 6, 2023 -
US hospitals forecast 9% capex growth to support elective procedure backlog: survey
The Baird survey of 38 health system CFOs forecasts that hospital capital spending growth in 2024 will slow from a 13% jump this year.
By Nick Paul Taylor • Dec. 6, 2023 -
J&J hernia mesh settlement prompts judge to dismiss more than 200 cases
The company and claimants in Georgia filed to dismiss cases related to Ethicon’s Physiomesh in late November.
By Nick Paul Taylor • Dec. 6, 2023 -
Intuitive’s venture capital arm launches second investment fund
The $150 million fund will back early-stage companies focused on healthcare access, digital health, and precision diagnostics and interventions.
By Susan Kelly • Dec. 5, 2023 -
Laboratory trade group, providers oppose FDA’s lab developed test proposal
A CDRH spokesperson confirmed to MedTech Dive that a discrepancy in the submission system used for the proposed rule led to an initial overcount of public comments.
By Nick Paul Taylor • Updated Dec. 7, 2023