Medical Devices: Page 28
-

 Retrieved from Exactech on March 14, 2024
Retrieved from Exactech on March 14, 2024
Exactech hit with warning letter over implant packaging
The FDA found faults in the orthopedics company’s analysis of complaints demonstrating defective packaging could have accelerated wear to its implants.
By Elise Reuter • March 14, 2024 -
Mass General Brigham works with FDA to create brain-computer interface group
The collaborators aim to resolve the clinical, regulatory, coverage and payment questions facing developers of the devices.
By Nick Paul Taylor • March 13, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
AI to expand medtech portfolios, revenue streams: Moody’s
The rating agency predicts AI will start to have a positive impact on medical device companies in the next two years.
By Nick Paul Taylor • March 12, 2024 -
3M’s board approves health spinoff
Investors will receive one share of Solventum for every four shares of 3M they hold on March 18.
By Nick Paul Taylor • March 12, 2024 -
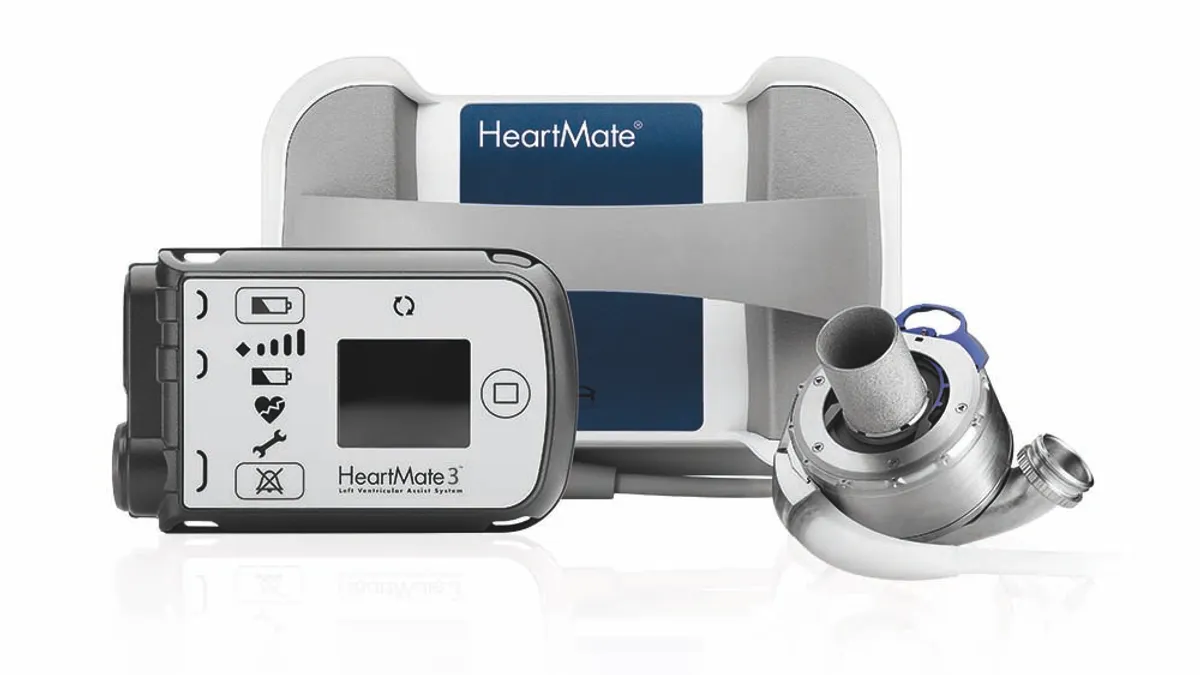
Abbott recalls Heartmate LVAD communication system
The FDA said eight reported injuries have been linked to the problem, which can cause the mechanical heart pump to unexpectedly stop or start.
By Susan Kelly • March 12, 2024 -
3M names William Brown CEO
Michael Roman, who has led 3M since 2018, will step down on May 1.
By Ricky Zipp • March 12, 2024 -
FDA panel backs Lumicell’s agent for breast cancer imaging tool
One panelist who voted yes said the “incremental benefits outweigh the small risks of anaphylaxis.”
By Nick Paul Taylor • March 11, 2024 -
Advamed report finds racial disparities in care, proposes fixes
The report found White patients were more likely to receive several cardiovascular procedures than Black patients, which limits access to medical technology.
By Nick Paul Taylor • March 11, 2024 -
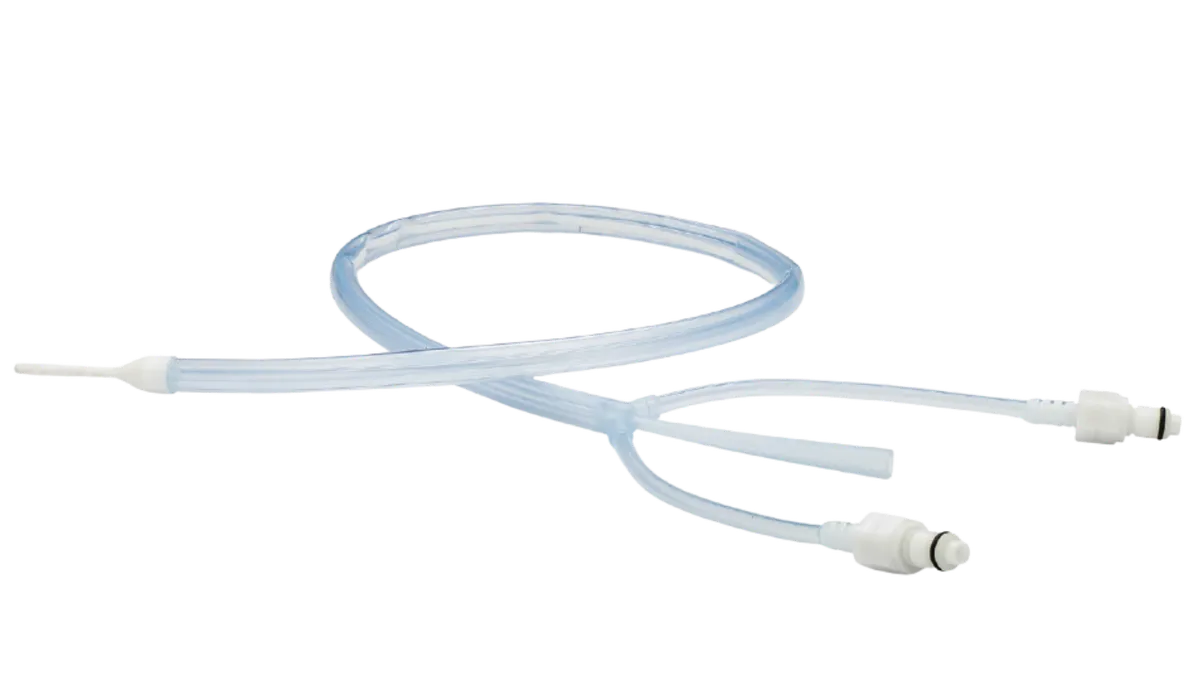
Medtronic recalls more than 45,000 catheter tubing units after injury reports
The issue, which is linked to 26 injuries, could result in neurological harm or death, FDA said.
By Nick Paul Taylor • March 8, 2024 -
Former Stimwave CEO found guilty of selling fake implantable device component
The former executive was convicted on two counts for her role in creating and marketing the faulty devices.
By Susan Kelly • March 8, 2024 -
Q&A
AI oversight is top challenge facing global device regulators: FDA official
Melissa Torres, CDRH’s associate director of international affairs, spoke about the importance of the International Medical Device Regulators Forum and how countries are struggling with AI oversight.
By Ricky Zipp • March 8, 2024 -
Siemens Healthineers launches anatomy hologram app on Apple Vision Pro
The prototype app has potential uses in patient communication, medical education and surgical planning.
By Nick Paul Taylor • March 8, 2024 -
Dexcom’s over-the-counter nod sets stage for broader CGM use
The FDA’s decision allows people who don’t take insulin, including those who don’t have diabetes, to use the devices without a prescription.
By Elise Reuter • March 7, 2024 -
Roche to take on Abbott, Dexcom with its first CGM
The new sensor is designed to last for 14 days but requires finger-stick calibration.
By Nick Paul Taylor • March 7, 2024 -
Baxter to close Massachusetts site, lay off 59 people
News of the cuts comes 13 months after the company outlined plans to eliminate 3,000 positions.
By Nick Paul Taylor • March 7, 2024 -
Vicarious presses forward with soft tissue robot despite struggles
The company detailed upcoming milestones for its robotic surgery system on a fourth-quarter earnings call.
By Susan Kelly • March 6, 2024 -
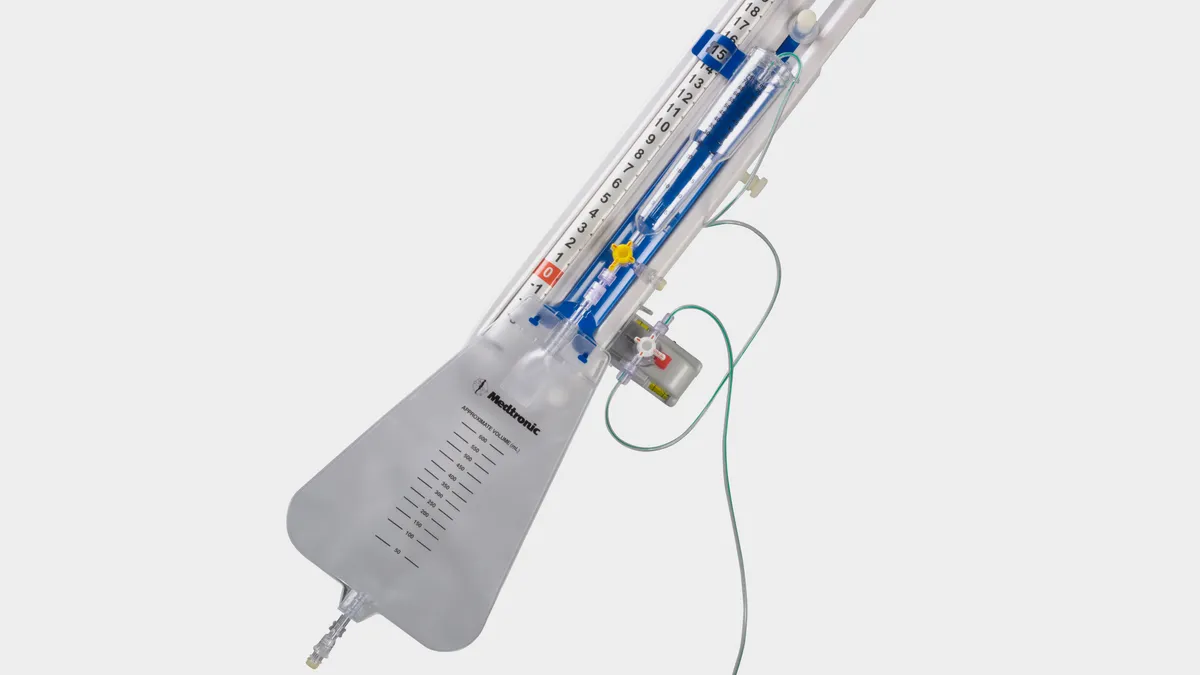
Smiths Medical recalls nearly 86,000 syringe pumps over software problem
The company fixed the issues, which are linked to one reported injury, by updating its software.
By Nick Paul Taylor • March 6, 2024 -
Dexcom receives FDA clearance for first OTC glucose sensor
The diabetes tech firm is tailoring its software to the 25 million people in the U.S. who have Type 2 diabetes and do not use insulin.
By Nick Paul Taylor • March 6, 2024 -
Haemonetics to buy Attune Medical for $160M
Attune’s device to help cool the esophagus during radiofrequency ablation procedures is a key piece of the acquisition.
By Susan Kelly • March 5, 2024 -
BD starts trial of vascular covered stent in peripheral arterial disease
Peripheral vascular disease is one of six key platforms BD is targeting for growth.
By Nick Paul Taylor • March 5, 2024 -
Medtronic estimates up to 40 job losses in Ireland due to restructuring
The company confirmed the potential job reductions from shutting down its ventilator product lines in an email to MedTech Dive.
By Ricky Zipp • March 5, 2024 -
Dexcom, Novo Nordisk call for FDA input on digital diabetes detection devices
The companies want clarity on what evidence would be needed for new technologies to detect undiagnosed Type 2 diabetes or prediabetes.
By Nick Paul Taylor • March 4, 2024 -
Baxter considers selling kidney care unit to private equity
The company expects to divest its largest segment, whether through a spinoff or a sale, in the second half of the year.
By Elise Reuter • March 4, 2024 -
GE Healthcare recalls incubators due to risk of newborns falling
The Food and Drug Administration labeled the recall as a Class I event after a serious injury was reported.
By Nick Paul Taylor • March 4, 2024 -
Boston Scientific gains FDA nod for drug-coated coronary balloon
BTIG analyst Marie Thibault said physicians could rapidly adopt the first-of-its-kind device.
By Susan Kelly • March 1, 2024