Medical Devices: Page 19
-
Deep Dive
Medtech firms have cut more than 14,000 jobs in the past 18 months
Diagnostics is the largest industry represented in the data, with more than 5,000 affected positions, according to MedTech Dive’s analysis.
By Jasmine Ye Han , Ricky Zipp , Elise Reuter • Updated July 25, 2024 -
Qiagen to cut 175 employees, close Michigan PCR test plant
The layoffs and plant closure are part of a restructuring plan that Qiagen estimates will cost about $400 million.
By Elise Reuter • July 17, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
J&J medtech fails to meet growth expectations
CFO Joe Wolk told investors Wednesday that Johnson & Johnson’s medical device business missed the company’s 5%-7% growth forecast in the second quarter.
By Ricky Zipp • July 17, 2024 -
Masimo sues activist investor Politan, postpones annual meeting
The latest turn in the recent drama comes days after proxy advisory firms ISS and Glass Lewis backed two Politan nominees for Masimo’s board.
By Susan Kelly • July 16, 2024 -
FDA creates communication ‘super office’ at CDRH
The changes are part of a broader reorganization intended to increase organizational agility and help the agency meet user fee commitments.
By Nick Paul Taylor • July 16, 2024 -
J&J, Abbott and Intuitive start medtech earnings season
The companies’ financial results will offer a glimpse at the current health of medtech markets.
By Nick Paul Taylor • July 16, 2024 -
Edwards to acquire Innovalve, stake in Affluent Medical
The heart valve specialist said in a federal filing that it expects to pay $300 million in cash for Innovalve Bio Medical when the deal closes.
By Susan Kelly • July 15, 2024 -
Hamilton recalls ventilators that may fail to restart
One death and one injury have been tied to the safety problem, the Food and Drug Administration said in a recall notice.
By Nick Paul Taylor • July 15, 2024 -
Element raises $277M to challenge Illumina in sequencing market
The startup claims companies that choose its device over Illumina’s sequencer can save almost $1.5 million in three years.
By Nick Paul Taylor • July 12, 2024 -
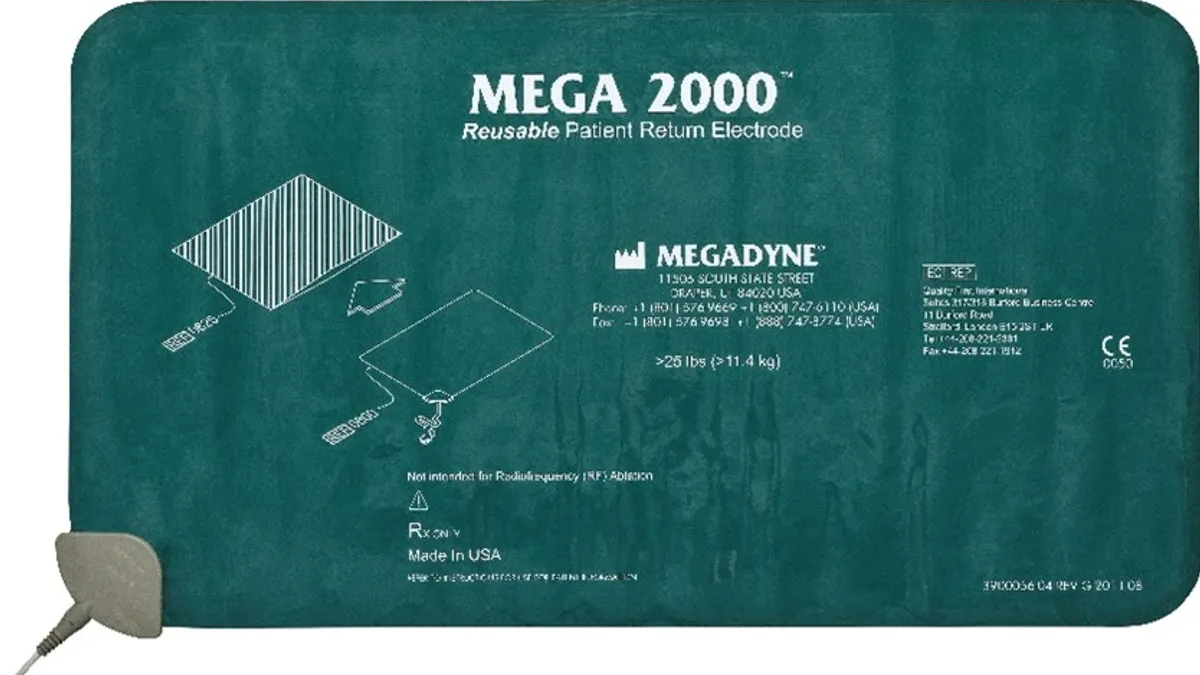
 Retrieved from Food and Drug Administration.
Retrieved from Food and Drug Administration.
J&J limits use of Megadyne electrodes due to burn risk
The FDA said 15 injuries have been tied to the use of three models of Megadyne patient return electrodes.
By Elise Reuter • July 12, 2024 -
FDA finalizes guidance on studying opioid use disorder treatments
The Center for Devices and Radiological Health is aiming to help companies manage challenges such as inaccurate participant reports of drug use.
By Nick Paul Taylor • July 12, 2024 -
Deep Dive
FDA’s lab developed test rule could be first check on agency’s power post-Chevron
The Supreme Court’s decision to overturn the Chevron doctrine would make it easier to challenge agency regulations, such as the LDT final rule.
By Susan Kelly , Elise Reuter • July 11, 2024 -
FDA warns BD blood culture bottle shortage could impact diagnosis
BD said reduced availability of blood culture media bottles due to supplier issues has prevented it from meeting global demand.
By Nick Paul Taylor • July 11, 2024 -
Medtronic recalls endotracheal tubes over blockage risk
The Food and Drug Administration has told healthcare providers to stop using the two affected types of endotracheal tubes.
By Nick Paul Taylor • July 11, 2024 -
Federal officials step down from health AI group’s board
HHS officials Micky Tripathi and Troy Tazbaz resigned from their roles as non-voting members of the Coalition for Health AI, an industry group working to create standards for artificial intelligence in healthcare.
By Emily Olsen • July 10, 2024 -
Roche wins CE mark for its first CGM
The clearance positions Roche to challenge Abbott and Dexcom for the European continuous glucose monitor market.
By Nick Paul Taylor • Updated July 10, 2024 -
Pulse drops 510(k) plan for PFA device after FDA requests clinical data
Analysts said the AFib device is now unlikely to come to market before late 2026.
By Nick Paul Taylor • July 9, 2024 -
Philips recall of imaging coils tied to 12 injuries
Several models of coils used in MRI scanners were recalled due to the risk of overheating, which can cause serious burns.
By Ricky Zipp • July 9, 2024 -
Masimo offered up to $950M for consumer business
Final terms of the deal are still being discussed, and Masimo said it plans to negotiate a higher price.
By Nick Paul Taylor • July 9, 2024 -
Baxter considers sale of kidney care unit to PE firm: WSJ
Carlyle Group could reportedly pay more than $4 billion for Baxter’s Vantive business.
By Elise Reuter • July 8, 2024 -
BD to close Ireland facility, putting 170 jobs at risk
A company spokesperson said the decision follows a recent review and reflects a push to improve operational efficiency.
By Nick Paul Taylor • July 8, 2024 -
Masimo employees rally around CEO Kiani as proxy fight looms
Hundreds of healthcare engineers have joined managers to support founder and CEO Joe Kiani as he battles an activist shareholder for control of the company.
By Susan Kelly • July 3, 2024 -
Alcon buys Belkin Vision for $81M upfront
Alcon will gain access to a laser treatment for glaucoma that it plans to sell in the U.S. by the end of the year.
By Nick Paul Taylor • July 3, 2024 -
Merit Medical pays Endogastric $105M for acid reflux treatment
Merit expects the device to add around $30 million in annual sales.
By Nick Paul Taylor • July 2, 2024 -

 Retrieved from Cardinal Health on July 02, 2024
Retrieved from Cardinal Health on July 02, 2024
FDA continues crackdown on plastic syringes made in China
The agency has issued import bans for four manufacturers, and multiple companies have recalled affected syringes. Check out MedTech Dive’s roundup of the news.
By Elise Reuter • July 2, 2024