Medical Devices: Page 173
-
DHS warns of critical cybersecurity weakness with Medtronic implants
The communication system vulnerability, in theory, could enable a hacker with limited skill to change settings on defibrillator implants.
By Nick Paul Taylor • March 22, 2019 -
Key senators demand answers about 'suspect' doctor-device maker ties
Bipartisan leaders of the Senate Finance Committee want CMS to probe whether physician-owned distributorships of medical implants are failing to meet Sunshine Act requirements.
By Nick Paul Taylor • March 22, 2019 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
FDA takes hard look at breast implant safety amid fresh cancer fears
The potential for the devices to cause autoimmune reactions may also be a talking point at Monday and Tuesday's advisory committee meeting. FDA called out potential biocompatibility problems with silicone last week.
By Maria Rachal • March 21, 2019 -
FDA, NEST detail how extra funding can bolster postmarket safety
CDRH Director Jeff Shuren explained the thinking behind the FDA's support of its postmarket surveillance system in a paper with Rachael Fleurence, executive director of the National Evaluation System for health Technology.
By Nick Paul Taylor • March 21, 2019 -
UK steps up no-deal planning as Brexit clock ticks down
MHRA updated its advice on registering devices against a backdrop of concerns about supply disruptions.
By Nick Paul Taylor • March 21, 2019 -
Alzheimer's device De Novo on the line at FDA panel
An advisory committee got started on a transcranial magnetic stimulation device Thursday morning, coming the same day as Biogen halted trials of one of the last late stage drug candidates to treat the disease.
By Susan Kelly • March 20, 2019 -
FDA warns Sientra, J&J's Mentor on lax post-approval breast implant studies
The action comes a week before the agency is set to hold a public meeting on risks related to the controversial devices.
By Susan Kelly • Updated March 20, 2019 -
Sponsored by ZS
Making software a core part of your business model
Pivoting away from devices as the primary revenue stream is proving to be a big challenge for many medtech companies.
By Dan Frey • March 19, 2019 -
BD wins PMA for stent treating venous occlusive disease
The company beat several contenders for the distinction of being first to market in the U.S. with a stent to reopen blocked iliac and femoral veins.
By Susan Kelly • March 19, 2019 -
FDA outlines new device priorities in budget justification report
A new agency document sheds light on proposed initiatives related to cybersecurity, approval pathways and special controls.
By Susan Kelly • March 19, 2019 -
FDA cybersecurity guidance draws fire from big device makers
Boston Scientific, GE Healthcare and BD are among manufacturers raising alarms over a proposal to create two tiers of cybersecurity risk buckets.
By David Lim • March 19, 2019 -
Breast implants, metal hips and other device materials under new FDA scrutiny
Symptoms such as fatigue, rash, joint and muscle pain or weakness may not appear until years after a device is implanted, FDA said.
By David Lim • March 18, 2019 -
JDRF-backed Korean medtech gets breakthrough nod for closed loop system
EOFlow is developing a closed loop automated insulin delivery system for people with Type 1 diabetes as more companies aim to introduce alternatives to Medtronic's 670G MiniMed system.
By Maria Rachal • March 18, 2019 -
FDA: Avoid paclitaxel-coated PAD devices for most patients
BD Thursday joined Medtronic in pushing back against the agency's findings, saying it stands behind the safety of the Lutonix drug-coated balloon.
By Susan Kelly • Updated March 22, 2019 -
TAVR called 'game changing' in studies of low-risk patients
Data from Edwards Lifesciences' Partner 3 trial and Medtronic's Evolut Low Risk study prompted predictions that TAVR will become the preferred option for a wider population of heart valve patients.
By Susan Kelly • March 18, 2019 -
Intuitive's da Vinci SP gets FDA otolaryngology clearance
The approval is the second procedure category to be cleared for the da Vinci single port system, as the company looks to grow internationally.
By Susan Kelly • March 18, 2019 -
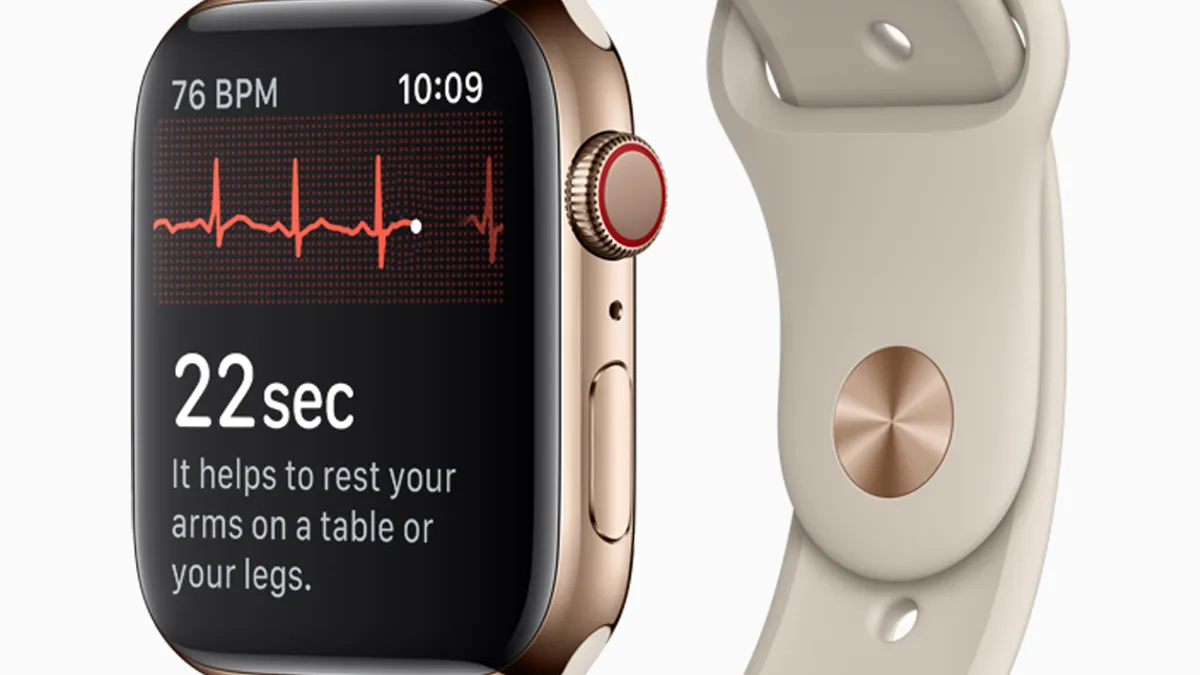
 Retrieved from Apple on September 12, 2018
Retrieved from Apple on September 12, 2018
Apple heart study: Early proof of concept, or a gimmick?
Doctors at the American College of Cardiology meeting said the Apple Watch's foray into health monitoring needs more data to prove its value.
By David Lim • March 16, 2019 -
Abbott gains expanded indication for MitraClip valve repair device
M&A has dominated the mitral space in recent years as structural heart competitors look to follow MitraClip's growth.
By Susan Kelly • March 15, 2019 -
FDA greenlights device for treating carbon monoxide exposure
The De Novo-winning Thornhill device accelerates a patient’s breathing rate to get poison out of the body.
By Nick Paul Taylor • March 15, 2019 -
Fresenius gets breakthrough status for hemodialysis software
The experimental software makes recommendations to improve fluid management and thereby cut the rate of complications.
By Nick Paul Taylor • March 15, 2019 -
Apple, Medtronic and more medtech to track at ACC19
Anticipated data backing the tech giant's heart rhythm-tracking watch, results from Medtronic and Edwards aimed at broader use of TAVR procedures, and an update on Abbott's MitraClip highlight the annual conference.
By Maria Rachal • March 14, 2019 -
Stryker to spend up to $220M on rotator cuff tear tech
The Kalamazoo, Michigan-based medtech announced it has closed a deal with Israeli startup OrthoSpace, which formerly drew investment from Johnson & Johnson and Smith & Nephew.
By Maria Rachal • March 14, 2019 -
HemoSonics gets De Novo nod for POC coagulation device
The test uses ultrasound to quickly analyze blood samples at the point of care.
By Nick Paul Taylor • March 14, 2019 -
Navigating FDA's evolving device pathways: A primer
The agency is touting the biggest alterations to medtech approval processes in the decades since it began reviewing devices. Here's a roundup of the current regulatory framework and what's potentially changing.
By Meg Bryant • March 13, 2019 -
FDA qualifies test for traumatic brain injury
The OsiriX CDE test can now be used by medical device developers to identify and enroll patients in studies of TBI, which has been researched for years but has no effective treatment.
By Susan Kelly • March 13, 2019




