Medical Devices: Page 164
-
Smith & Nephew completes meniscus repair acquisition
The company said Tuesday its $50 million purchase of Ceterix Orthopaedics has closed, with additional payouts up to $55 million possible over the next five years based on the associated meniscal repair system's financial performance.
By Maria Rachal • Updated Jan. 23, 2019 -
Aortic stent graft market on path to solid growth
Aortic stent grafts are on track to become a $4.5 billion global market over the next decade, expanding at a compound annual growth rate of 5.4% thanks to advances in product design, according to a forecast by GlobalData.
By Susan Kelly • Dec. 19, 2018 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Cardiva wins FDA approval for its vascular closure system
The Santa Clara, California-based company succeeded in its PMA application and can now market the Vascade MVP device for use in electrophysiology procedures.
By Susan Kelly • Dec. 19, 2018 -
Researchers advance 3D-printed, wirelessly-controlled ingestible
The multi-purpose device could spend a month or more in the stomach as a diagnostic tool or drug delivery mechanism.
By Nick Paul Taylor • Dec. 19, 2018 -
FDA outlines fast track vision for breakthrough devices
The agency also announced plans to establish a pathway for devices aimed at treating non-life-threatening diseases not eligible for the original program.
By David Lim • Dec. 18, 2018 -
Medtronic wins FDA OK for InterStim smart programmer
The device giant also got its Navion stent graft system to be included in an aneurysm repair study with Aortica software.
By Susan Kelly • Dec. 18, 2018 -
FDA addresses manufacturing site changes in final guidance
The agency issued final guidance detailing when a medical device maker that plans a change in manufacturing site should submit a premarket approval application supplement.
By Susan Kelly • Dec. 17, 2018 -
Nonprofit gets boost to realize automated insulin delivery app
The software is meant to work with multiple insulin pumps and continuous glucose monitoring devices and will be designed for the iOS App Store as an FDA-regulated mobile application, said diabetes technology nonprofit Tidepool.
By Susan Kelly • Dec. 17, 2018 -
FDA walks back new device class definitions after industry blowback
AdvaMed and the Medical Device Manufacturers Association scored a temporary win after arguing the proposed revisions to the Class III definition could shift more devices into the high-risk category.
By David Lim • Dec. 14, 2018 -
Medtech industry cheers as NAFTA 2.0 edges forward
Trade groups heralded improved transparency in the recently-signed USMCA, which includes a provision that would enforce more timely reimbursement decision-making by national bodies.
By Nick Paul Taylor • Dec. 14, 2018 -
FDA touts progress in move toward global device audits
Participating manufacturing sites have spiked this year with 2,629 cumulatively added as of the third quarter of 2018, up from 777 at the end of 2017.
By David Lim • Dec. 13, 2018 -
FDA adds information on device BLAs to user fee guidance
The revised document features additional information on the fees for device biologics license applications.
By Nick Paul Taylor • Dec. 13, 2018 -
FDA rates GE ventilator safety guard recall as Class I event
The recall was triggered after the tech giant discovered the device has the potential to disconnect from the patient's breathing circuit.
By Nick Paul Taylor • Dec. 13, 2018 -
Apple EKG, artificial iris among FDA 2018 device decisions
De Novos are on the rise, but both premarket approvals and 510(k)s appear to be slightly down from 2017, according to nearly year-end data from FDA.
By Meg Bryant • Dec. 12, 2018 -
Boston Scientific scores US victory in TAVR patents battle
A jury in the U.S. District Court for Delaware determined that the Sapien 3 device from Edwards Lifesciences infringed a Boston Scientific patent in the hotly contested transcatheter aortic valve replacement systems market.
By Maria Rachal • Dec. 12, 2018 -
UK readies 'worst-case' Brexit plan for medical devices
The government issued an update on its contingency planning if no deal is reached by March 29 for the country to remain part of the EU medicines and medical devices regulatory networks.
By Susan Kelly • Dec. 12, 2018 -
HIMSS: Health coaching could boost value of wearables
HIMSS surveyed 101 health IT and hospital administrators for the Fitbit-sponsored report, which found that half of hospitals and health systems were already implementing health coaching, or planned to do so.
By Susan Kelly • Dec. 12, 2018 -
Hospital beds from Hill-Rom get sensor tech
Sensors continually check patients' heart and respiratory rates and alert providers if their vital signs change.
By Meg Bryant • Dec. 11, 2018 -
Hologic begins selling 3-in-1 uterine scope
The device is intended to improve the procedure for treating uterine conditions by avoiding the need to change from a diagnostic to an operative scope.
By Susan Kelly • Dec. 11, 2018 -
Duodenoscope studies find higher-than-expected contamination rate, FDA says
Across studies run by Olympus, Fujifilm and Pentax, 6% of sampled devices were contaminated with microorganisms including pathogenic strains such as E. coli.
By Nick Paul Taylor • Dec. 11, 2018 -
Cybersecurity priorities laid out by key House panel
The House Energy and Commerce Committee issued a report outlining six strategies for strengthening the nation's defenses against cybersecurity vulnerabilities with a focus on medical devices in healthcare.
By Susan Kelly • Dec. 10, 2018 -
FDA clears Contego Medical peripheral angioplasty system
The catheter-based device for use in procedures to treat peripheral artery disease incorporates an adjustable filter to capture blood clots and is intended for high-risk patients.
By Susan Kelly • Dec. 10, 2018 -
FDA to revisit transvaginal mesh issues at February meeting
The agency said it may consider additional regulatory actions based on public feedback and Feb. 12 advisory panel discussions.
By Susan Kelly • Dec. 10, 2018 -
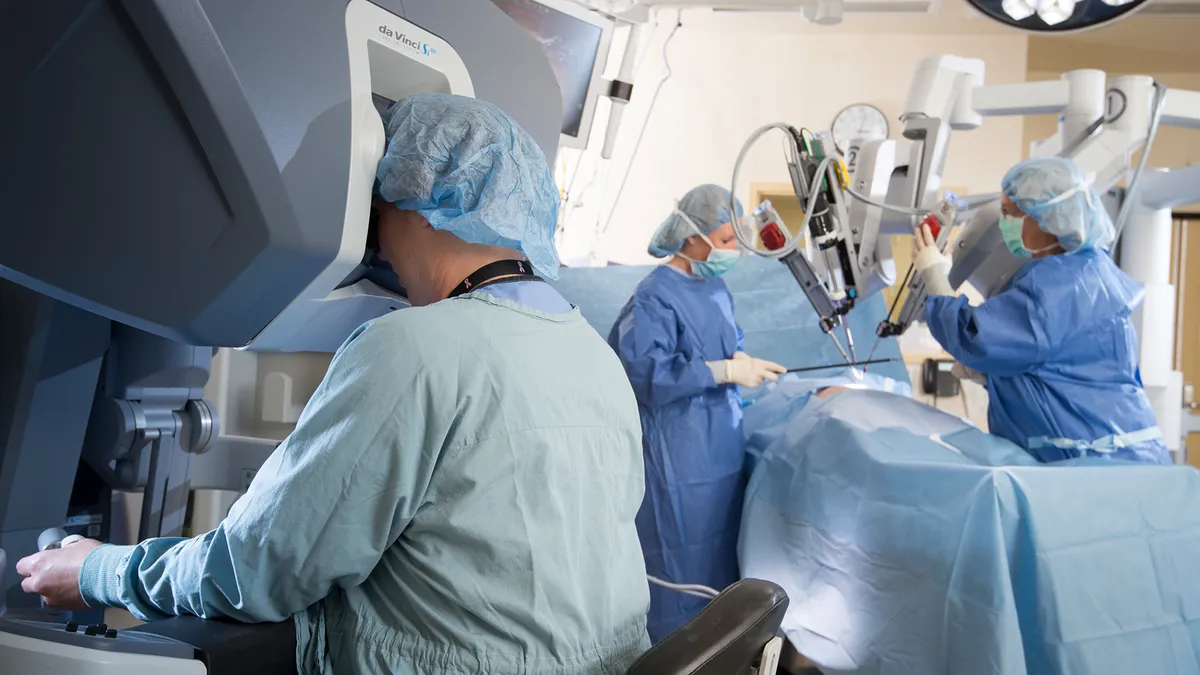
UPenn surgeons perform first robot-assisted bilateral breast reconstruction
The physicians used Intuitive's da Vinci system to increase the precision of the minimally-invasive procedure.
By Nick Paul Taylor • Dec. 7, 2018 -
Device industry 2019 outlook beats hospitals, pharma: Moody's
The credit ratings agency said the sector will benefit from new product innovation and demand from emerging markets, but could face headwinds from rising trade tensions and the reinstatement of the medical device tax in 2020.
By Susan Kelly • Dec. 7, 2018