Medical Devices: Page 152
-
Boston Scientific Q1 hit by woes over mesh, paclitaxel, sterilization
The company lowered full year revenue guidance and slashed expectations for its Eluvia paclitaxel-eluting stent by 50% ahead of an FDA advisory panel.
By Maria Rachal • Updated April 25, 2019 -
FDA pitches stronger controls, new labeling for surgical staplers
The proposed order follows an agency review that found the widely used tools were linked to numerous problems and serious complications, including 366 deaths, over a seven-year period.
By Susan Kelly • April 24, 2019 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Edwards Lifesciences beats profit forecasts amid low-risk TAVR momentum
The structural heart-focused company posted close to $1 billion in first quarter sales, exceeding Wall Street expectations.
By Susan Kelly • April 24, 2019 -
CMS floats new add-on payment route for breakthrough devices
The agency's proposed rule for 2020 outpatient payments pitches a similar alternative pathway aimed at speeding coverage for select medical technologies.
By David Lim • Updated July 31, 2019 -
Playing catch up, Boston Scientific gets FDA approval for Lotus TAVR device
The U.S. OK comes 30 months after an issue with the delivery system locking mechanism led Boston Scientific to stop installations of the product in Europe.
By Nick Paul Taylor • April 24, 2019 -
Surgical instruments lead organic growth for Stryker in Q1
Despite the beat-and-raise start to 2019, Stryker posted second quarter earnings guidance that fell short of expectations.
By Nick Paul Taylor • April 24, 2019 -
Study supports genetic testing for sudden cardiac arrest
The research, published in the Journal of Cardiology, found the tests to be useful regardless of whether patients showed previous clinical evidence of heart disease.
By Susan Kelly • April 23, 2019 -
FDA advisory panels set for surgical staplers, paclitaxel devices
Regulators are seeking expert advice regarding paclitaxel-coated devices in peripheral artery disease patients, and on surgical staplers, which FDA suggests should be reclassified after a spike in adverse event reports.
By Maria Rachal • April 23, 2019 -
Obesity device to slow stomach emptying gets approval
BaroNova's clinical study met its primary endpoints for percent total body weight loss 12 months after the procedure.
By Susan Kelly • April 23, 2019 -
More patients die after starting dialysis than registry suggests: Harvard study
The death rates for dialysis patients in a new JAMA Internal Medicine analysis were almost twice as high as mortality statistics from the U.S. Renal Data Registry.
By Susan Kelly • April 23, 2019 -
Trade groups seek to guide suppliers past pitfalls of new CMS bidding process
The new lead item bidding model could lead to significant reductions in Medicare rates for medical equipment.
By Nick Paul Taylor • April 23, 2019 -
USMCA would boost medtech industry, ITC report finds
AdvaMed urged Congress to ratify the agreement "as expeditiously as possible," arguing the International Trade Commission report demonstrates it would be an improvement over the North American Free Trade Agreement.
By David Lim • April 22, 2019 -
FDA wants details on nitinol in premarket device applications
The agency aims to gather more thorough information from manufacturers in its effort to gauge the widely used metal's potential to corrode or cause allergic reactions.
By Susan Kelly • April 22, 2019 -
FDA drafts premarket recommendations for quantitative medical imaging
Data obtained through the technologies can aid in patient diagnosis, prognosis and treatment, but FDA cautioned numerical values derived from the images may be subject to multiple sources of measurement error.
By Susan Kelly • April 22, 2019 -
CDC rule removes barrier to sale of escape respirators
The deregulatory action was inspired by concern that underground coal miners could face a dangerous shortage of the breathing devices, relied on as a means of survival in emergencies.
By Susan Kelly • April 22, 2019 -
FDA clears first medical device indicated for ADHD
The De Novo-winning nerve stimulation system, which can be administered at home in certain patients ages 7 to 12 years old, serves as an alternative to prescription medication in treating attention deficit hyperactivity disorder.
By Maria Rachal • April 22, 2019 -
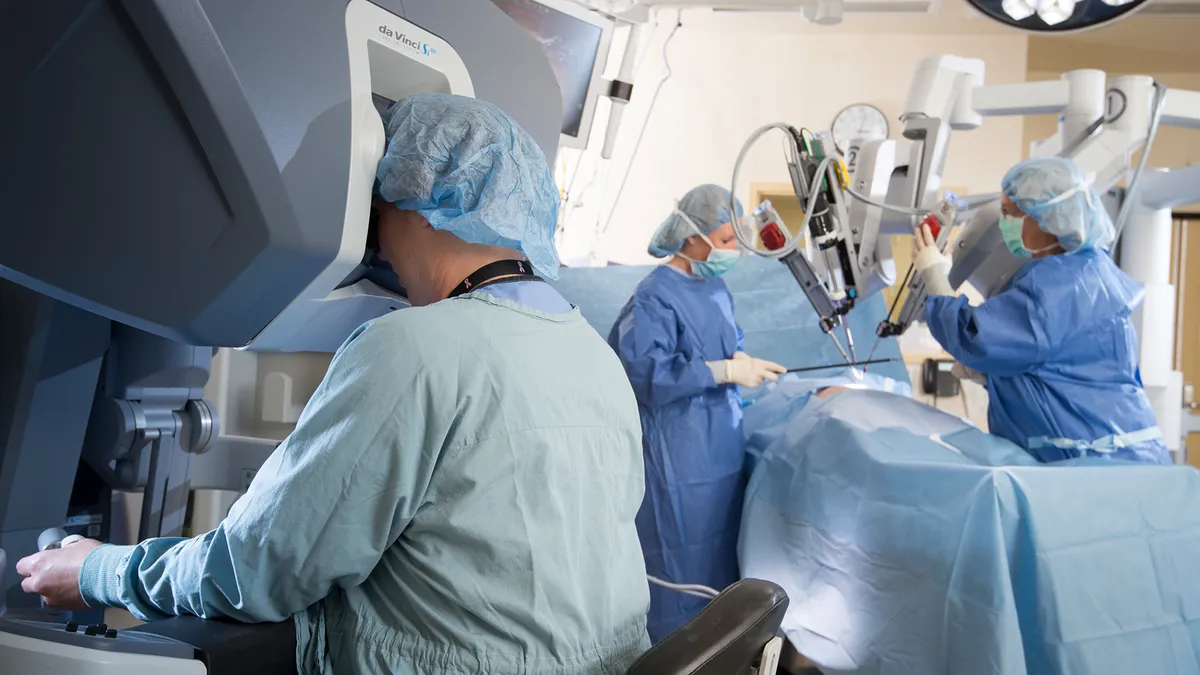
Intuitive shares tumble as Q1 earnings miss analyst expectations
The robotic surgery leader reported double-digit growth in procedures, shipments of surgical systems and sales, but the earnings miss reflected rising spending and lower revenues resulting from a shift to leasing.
By Nick Paul Taylor • April 22, 2019 -
Best Buy, Target jump into at-home device market
The partnership is an example of companies reaching customers through mass retailers, tightening the relationship between healthcare and retail.
By Daphne Howland • April 18, 2019 -
FDA grants breakthrough status to preeclampsia device
Advanced Prenatal Therapeutics designed the device to remove pathogenic factors from a mother's blood, seeking to prevent a pregnancy condition that can be fatal.
By Nick Paul Taylor • April 18, 2019 -
WHO weighs in on digital health tools in new report
The World Health Organization reviewed 10 digital health interventions, backing use of mobile devices for functions like clinical decision support and telemedicine. Still, a top official said the strategies are "not a silver bullet."
By Nick Paul Taylor • April 18, 2019 -
Medtronic posts data on stent grafts and venous closure systems
The endosuture aneurysm repair and chronic venous disease data suggest the efficacy seen earlier in the studies is durable.
By Nick Paul Taylor • April 18, 2019 -
FDA orders Boston Scientific, Coloplast to pull transvaginal mesh from market
The medtechs have 10 days to submit a withdrawal plan, two months after an agency advisory panel noted a worrisome lack of long-term safety and effectiveness data for the much-litigated pelvic organ prolapse repair devices.
By Maria Rachal • April 17, 2019 -
Brainlab recalls spine 3D navigation software for incorrect display
The software could display inaccurate information during a procedure that may prevent the surgeon from safely navigating surgical tools inside the patient, causing serious injury or death.
By Susan Kelly • April 17, 2019 -
AI poised to 'transform' medical imaging, radiology society says
Earlier this month FDA outlined in a white paper its vision for a framework to regulate artificial intelligence algorithms that change based on real-world learning.
By Susan Kelly • April 17, 2019 -
Abbott beats guidance in Q1, ramps up full-year earnings forecast
The medical device unit led the way once again with 5.5% growth, largely fueled by fast-rising demand for the company's FreeStyle Libre continuous glucose monitor.
By Nick Paul Taylor • April 17, 2019