Medical Devices: Page 146
-
Glaukos strikes deal to buy retinal drug delivery company
The takeover will give Glaukos ownership of micro-invasive, sustained-released, bio-erodible drug delivery platforms.
By Nick Paul Taylor • June 21, 2019 -
SEC ends Misonix foreign corruption probe without action
The maker of ultrasonic devices triggered the investigation in 2016 when it contacted the SEC with information about the business practices of its Chinese distributor.
By Nick Paul Taylor • June 21, 2019 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
FDA safety panel pans lack of paclitaxel device postmarket data on Day 1
The agency's Circulatory System Devices Panel Advisory Committee agreed Wednesday there is a mortality signal associated with paclitaxel-coated balloons and paclitaxel-eluting stents.
By David Lim • June 20, 2019 -
Philips gets FDA approval for over-the-counter defibrillators
The devices were previously cleared for sale in the U.S. via the 510(k) pathway but now need PMA approval.
By Nick Paul Taylor • June 20, 2019 -
Bursting balloon catheters prompt Cook Medical recall
The action comes less than a year after the Bloomington, Indiana-based device maker resolved an FDA warning letter faulting its quality management system.
By Susan Kelly • June 19, 2019 -
Swiss notified body to exit sector, heightening EU capacity fears
QS Zürich’s withdrawal will leave Switzerland with one medtech notified body, as new rules for the bloc near.
By Nick Paul Taylor • June 19, 2019 -
FDA approves cochlear implant designed for MRI
The implant is designed for safe access to magnetic resonance imaging scans and doesn't require a head wrap or removal of the device's internal magnet.
By Susan Kelly • June 18, 2019 -
FDA safety panel to weigh paclitaxel-coated stents, balloons
The advisory panel, which will meet for two days starting Wednesday, will examine mortality and causality issues surrounding the paclitaxel-coated devices sold by BD, Medtronic, Cook Medical, Philips and Boston Scientific.
By David Lim , Maria Rachal • Updated June 18, 2019 -
As CMS decision on TAVR looms, a push for broader access
Proposed agency rules would lower the bar for hospitals performing the less invasive heart procedure, but would also raise the eligibility requirements to offer the treatment.
By Nick Paul Taylor • June 18, 2019 -
FDA 'white hat' hacking tools may combat medical misinformation
Cybersecurity techniques the agency uses to protect medical devices could also help fight the spread of medical misinformation, former FDA Commissioner Robert Califf wrote in a journal.
By Susan Kelly • June 17, 2019 -
Health Canada seeks advice on device action plan next steps
The agency is asking for public comments on proposed rule changes that would require device makers to provide post-market safety information about products.
By Susan Kelly • June 17, 2019 -
SaMD will increase device innovation: Fitch
FDA’s proposed regulatory framework will allow medical technology manufacturers to incorporate AI and machine learning while improving software monitoring.
By Susan Kelly • June 17, 2019 -
Margin for error 'almost zero' in testing pediatric devices
As barriers dog pediatric device development, one FDA-backed group is trying a different path.
By Maria Rachal • June 14, 2019 -
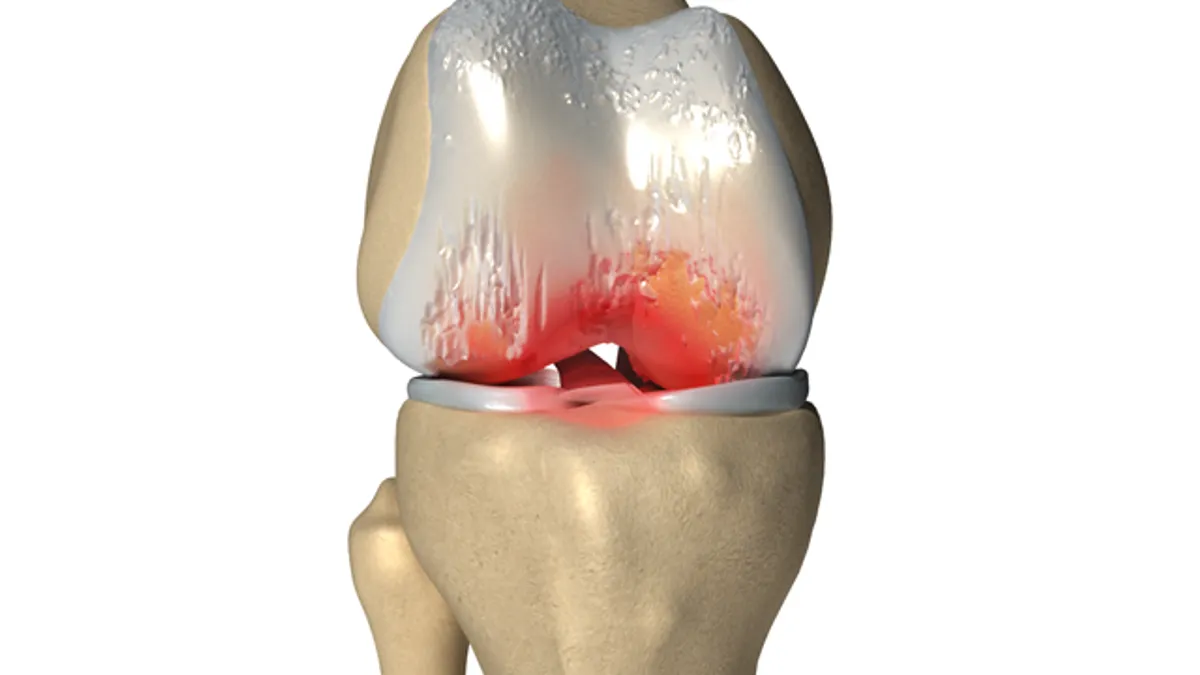
Many in need of knee cartilage repair excluded from studies, researchers say
More inclusive clinical trial designs would better reflect the broader population needing treatment, study authors say.
By Susan Kelly • June 14, 2019 -
Lloyd's exits notified body services, leaving UK with 3
The withdrawal comes as Europe faces major concerns about capacity ahead of the new MDR and IVD regs.
By Nick Paul Taylor • June 14, 2019 -
DHS warns of severe security flaw with BD infusion pumps
The department gave the BD Alaris Gateway Workstation vulnerability the maximum score on a standard grading scale.
By Nick Paul Taylor • June 14, 2019 -
AMA adopts measures to shape development, uptake of AI systems in healthcare
The policies seek to ensure developers of AI, not physicians, are liable for medical errors involving the technologies.
By Nick Paul Taylor • June 13, 2019 -
FDA posts draft guidance on testing of reproductive devices
The agency details how developers and manufacturers of assisted reproduction technology devices can perform mouse embryo assays to assess embryotoxicity.
By Nick Paul Taylor • June 13, 2019 -
Device maker to pay $15M to resolve false claims allegations, 'silent recall'
ACell failed to report a product recall to FDA and billed Medicare for indications sales reps knew were not backed by clinical data to receive inflated reimbursement, the Department of Justice said.
By David Lim • June 12, 2019 -
China leads world in digital health adoption, Philips survey finds
Healthcare professionals in China are more likely to recommend patients use digital health technologies than their peers in the West.
By Nick Paul Taylor • June 12, 2019 -
Germany, Ireland push EU to assess device regs preparedness
The delegations think a significant reduction in notified bodies is likely but hope to avoid delaying implementation "unless absolutely necessary."
By Nick Paul Taylor • June 12, 2019 -
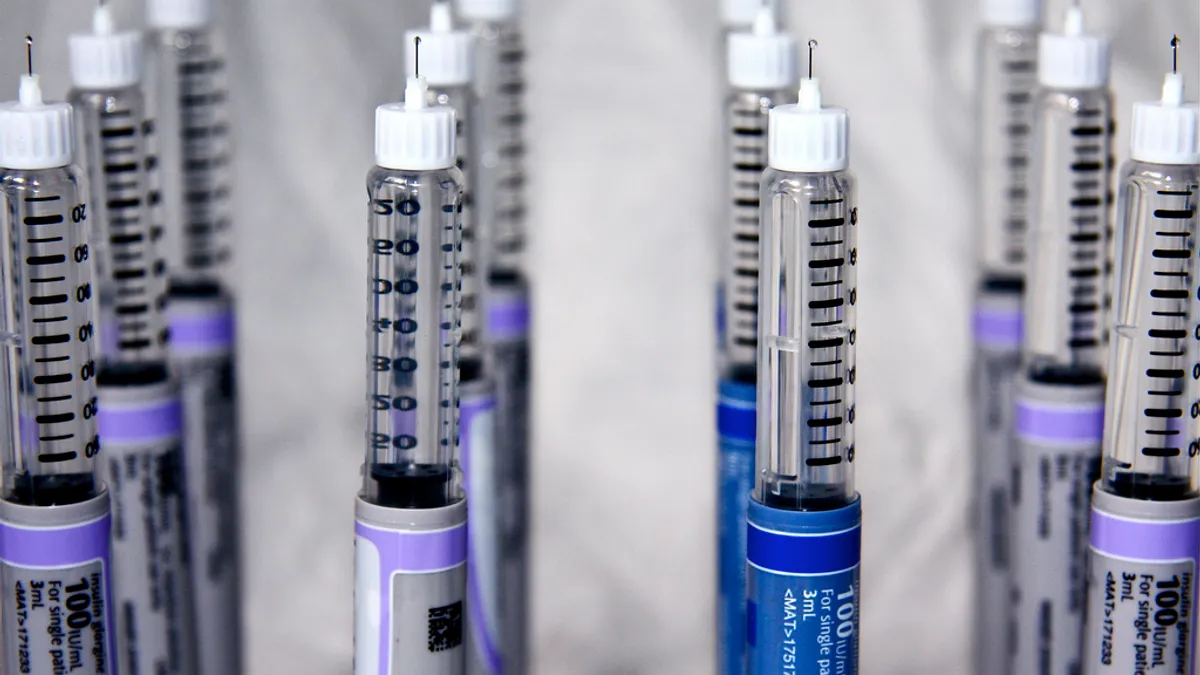
Beta Bionics, Insulet seek shake-up of insulin delivery market at ADA
Data presented at the American Diabetes Association meeting move the companies a step closer to challenging Medtronic and Tandem.
By Nick Paul Taylor • June 11, 2019 -
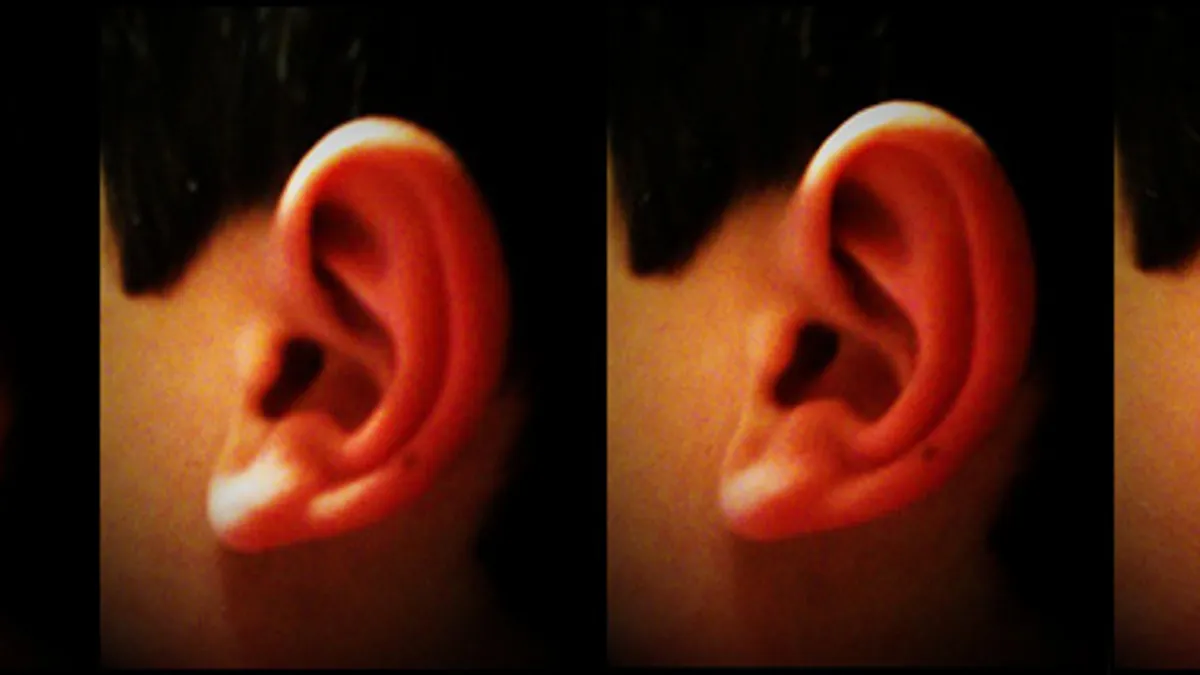
Stimulation device for IBS pain in children gains De Novo clearance
The non-invasive nerve stimulator is placed behind the patient’s ear to send electrical pulses to cranial nerve bundles.
By Susan Kelly • June 10, 2019 -
Medtech avoids tariff blow as US, Mexico reach deal
President Donald Trump late Friday called off plans for a 5% tariff set to go into effect today on imports from Mexico.
By Susan Kelly • June 10, 2019 -
Device interoperability, CGM for Type 2 patients among ADA 2019 standouts
More options for automated insulin dosing systems are emerging, particularly as FDA established new device classifications for interoperable pumps and glycemic controller algorithms via the De Novo pathway.
By Maria Rachal • June 10, 2019