Medical Devices: Page 144
-
FDA qualifies tool for nonclinical testing of ultrasound devices
The tool features a material designed to mimic the behavior of tissue to enable companies to assess and improve the safety of devices.
By Nick Paul Taylor • July 12, 2019 -
PMA approval rate has jumped since FDA changed panel meetings
The agency's likelihood of granting approval to PMA applications reviewed by an advisory committee rose from 70% to 92% after FDA changed in 2010 to a system where panelists vote anonymously on multiple questions.
By Nick Paul Taylor • July 12, 2019 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Abbott Alinity blood plasma screening system gets FDA nod
The machine is designed to increase efficiency at diagnostic screening laboratories, run more tests in less space, more quickly generate test results and minimize human error.
By Dana Elfin • July 11, 2019 -
Mandatory bundled radiation oncology demo seeks to upend payment
Manufacturers with the most exposure include Varian Medical Systems, Accuray, Elekta and Siemens, according to Cowen.
By David Lim • July 11, 2019 -
Baxter to expand home dialysis on heels of Trump kidney initiative
Amid a push to move away from costly dialysis centers, Baxter said it expects to add and bolster facilities for making and distributing peritoneal dialysis solutions, devices and cassettes.
By Maria Rachal • July 11, 2019 -
FDA finalizes IDE broadcasting guidance, with concessions to industry
The final text is a major rewrite of a 2014 draft, which was criticized by AdvaMed.
By Nick Paul Taylor • July 11, 2019 -
FDA hits Clinicon with warning letter over sterility failings
Inspectors found fault with the validation and calibration of processes and equipment involved in ensuring sterility of laser devices.
By Nick Paul Taylor • July 11, 2019 -
Hill-Rom to sell surgical consumables unit for $170M
The transaction will free Hill-Rom of a stagnant business and its 500 employees, while giving it more cash to spend on acquisitions.
By Nick Paul Taylor • July 11, 2019 -
Trump executive order seeks to overhaul US kidney care
The plan aims, in part, to increase the number of organs available for transplant by encouraging the development of artificial and wearable kidneys and modernizing the organ recovery and transplantation system.
By Rebecca Pifer • Updated July 10, 2019 -
USTR exempts some medical devices from 25% China tariffs
More decisions on pending requests for exemption will be made on a periodic basis, according to the U.S. Trade Representative.
By David Lim • July 10, 2019 -
Amazon's momentum in medical supply chain slowing, poll finds
A UBS survey of hospital purchasing managers found providers are allocating purchases of surgical tools, orthopaedic and cardiology implants, and other medical supplies to Amazon at a lower rate this year compared to 2018.
By Rebecca Pifer • July 10, 2019 -
Edwards poised for a Q2 TAVR rebound, analysts predict
Jefferies tracked hospital purchasing data and found U.S. transcatheter aortic valve replacement procedures with Edwards' devices likely accelerated in the second quarter, despite Boston Scientific adding new competition to the mix.
By Susan Kelly • July 10, 2019 -
Hospital purchasers call 3D mammography, robots and TAVR investment priorities
UBS estimates stable growth of 3.6% in hospital capital budgets based on data collected from managers surveyed.
By David Lim • July 9, 2019 -
The 10 biggest medtech stories of Q2
Key storylines — like escalating tariffs between the U.S. and China, FDA's evolving position on paclitaxel devices, and the uncertain capacity of Europe's notified bodies — will unfold in the second half of the year.
By Maria Rachal • July 9, 2019 -
Medtronic HVAD shows low stroke risk in study of thoracotomy approach
The clinical data could help Medtronic reverse market share losses to Abbott, which makes a competing left ventricular assist device for patients suffering from advanced heart failure.
By Susan Kelly • July 9, 2019 -
Feds warn of cyber vulnerability in hospital anesthesia machines
The issue, found in GE's Aestiva and Aespire devices, could allow an attacker to impair respirator functionality by silencing alarms, altering time and date records, and changing the composition of aspirated gases.
By Susan Kelly • July 9, 2019 -
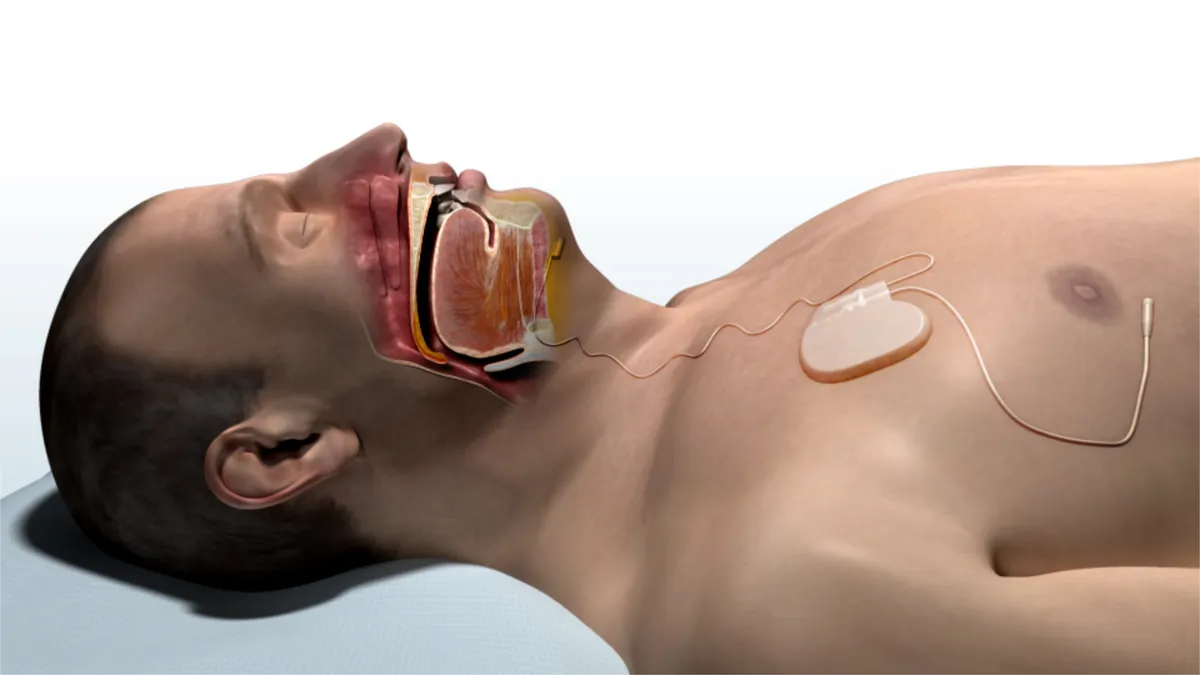
UnitedHealth backs Inspire Medical's sleep apnea implant
The massive private payer deemed the Minnesota medtech's neurostimulation system medically necessary in patients with obstructive sleep apnea who have already tried CPAP therapy.
By Maria Rachal • July 9, 2019 -
UL to close UK medical device notified body operation
The notified body is stepping back from the U.K. device sector altogether and will limit its in vitro diagnostic services in response to the risk of a no-deal Brexit.
By Nick Paul Taylor • July 9, 2019 -
Sens. Warren, Murray probe FDA's 'alarming' progressive device approval idea
The Democrats gave the agency until July 8 to respond to their questions on a recent budget proposal that seeks to grant FDA a two-step approval process for medical devices for life-threatening conditions.
By David Lim • July 8, 2019 -
Edwards issues safety notice for Centera valve after reports of injury, death
The company said difficulty manipulating the device around the aortic arch has resulted in reports of vascular injury, and recommended use of a stiffer guide wire or an alternate treatment for certain types of anatomies.
By Susan Kelly • July 8, 2019 -
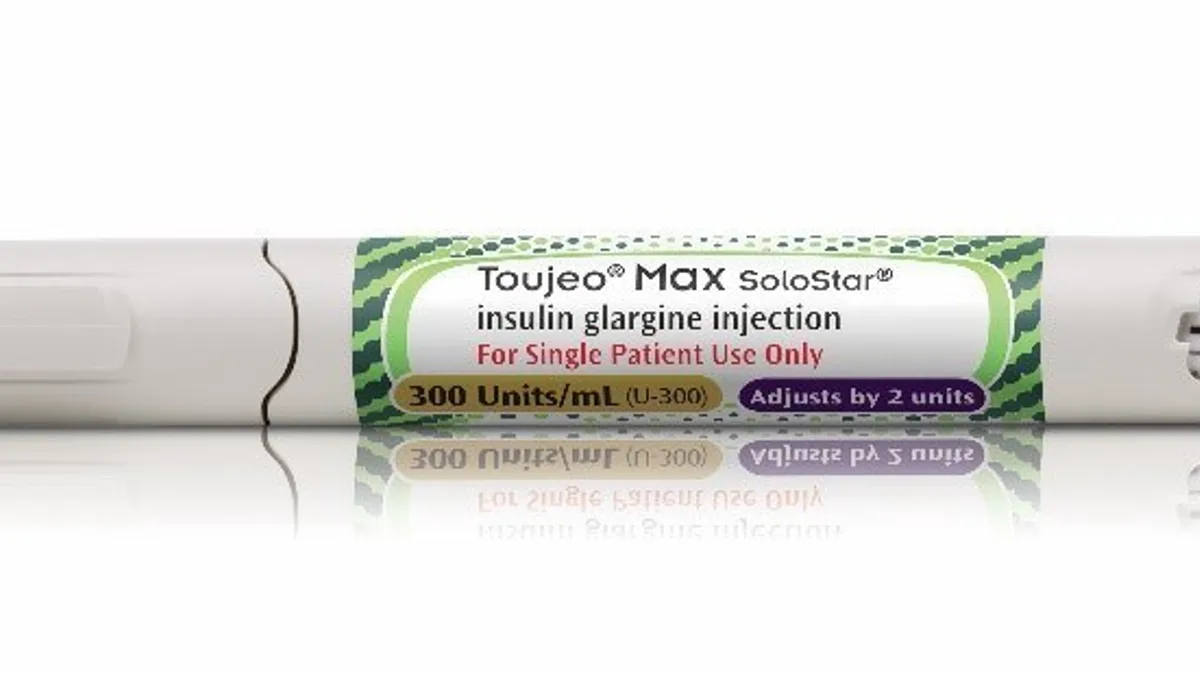
Insulin pen smart cap maker in talks to supply Sanofi
French medical device maker Biocorp's Internet-connected cap is designed to work with a conventional insulin pen and can automatically transmit dosage data to a smartphone app.
By Susan Kelly • July 8, 2019 -
Spanish agency steps up to become an MDR notified body
The decision comes as some in the sector have expressed concerns about whether there will be enough notified bodies to adequately support the transition to the EU's new medical device rules.
By Susan Kelly • July 8, 2019 -
Invacare lays off 75 as turnaround effort drags on
The medical equipment manufacturer is making cuts in Europe and North America, adding to the steady flow in both regions in recent years.
By Nick Paul Taylor • July 4, 2019 -
Doctors significantly over-allocated times to perform joint replacements, study shows
Time periods estimated to perform medical procedures, known as relative value units, are crucial for determining how much surgeons and other doctors are paid for their work under the Medicare Physician Fee Schedule.
By Ron Shinkman • July 3, 2019 -
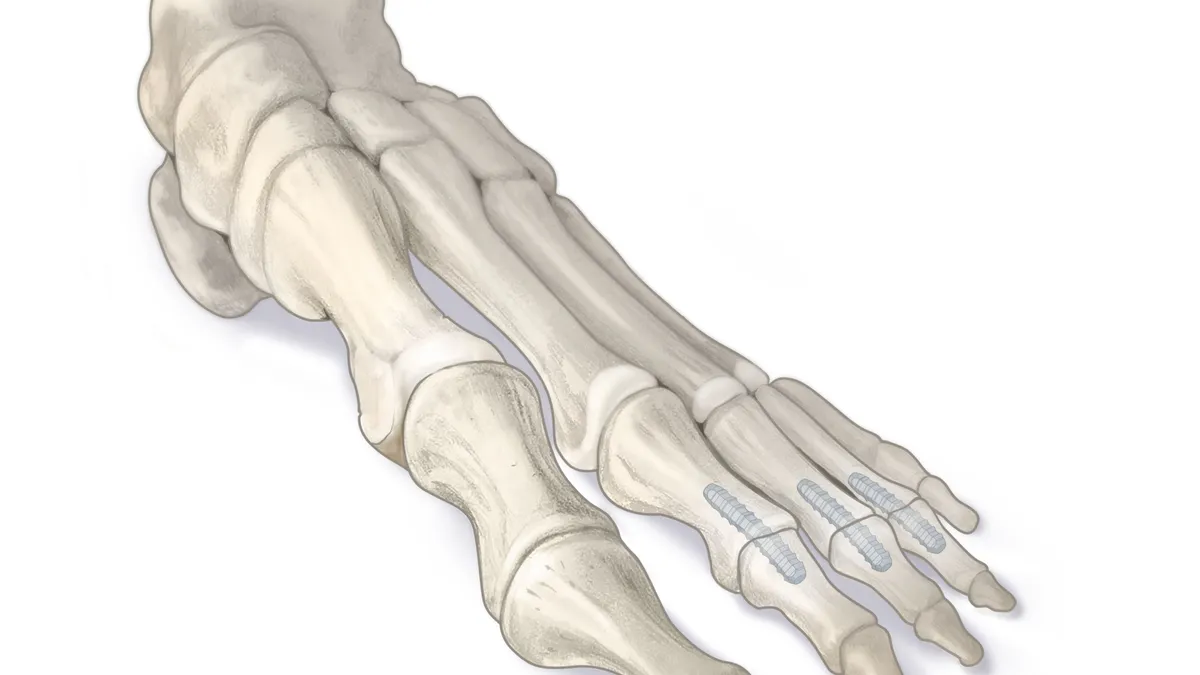
Wright Medical shares tumble amid report of Cartiva slowdown
Surgeons are implanting fewer of the devices to treat arthritis of the big toe as more patients report dissatisfaction with post-operative pain, degree of motion or device slippage, RBC Capital Markets analysts said in a research note.
By Susan Kelly • July 3, 2019