Medical Devices: Page 122
-
Senseonics cites Freestyle Libre pricing as factor in its Roche deal challenges
The implantable continuous glucose monitor maker forecast sales outside the U.S. to drop by one-third this year.
By Nick Paul Taylor • March 13, 2020 -
TransMedics stock falls double-digits as PMA meeting again delayed by FDA
The "unforeseen delay," according to the company's CEO, further pushes back the advisory panel review of the company's heart transplant device.
By Maria Rachal • Updated Sept. 29, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
FDA finalizes contentious guidance on third party 510(k) reviews
Meant to enable faster decisions and free up the agency to focus on higher risk devices, earlier versions took fire from AdvaMed and some patient groups.
By Nick Paul Taylor • March 12, 2020 -
Rival to Boston Scientific device used in TAVR wins CE mark
Keystone Heart, the Israel-based unit of Chinese structural heart player Venus Medtech, is readying an FDA submission for its cerebral embolic protection device, intended to reduce stroke risk during transcatheter heart procedures.
By Susan Kelly • March 11, 2020 -
Medtronic's HVAD controversy
FDA issues Class I recall for Medtronic's HeartWare HVAD after patient death
A design flaw flagged by Medtronic in January regarding its ventricular assist devices landed a high-risk label from regulators this week.
By Nick Paul Taylor • March 11, 2020 -
Key EU notified body meeting goes on, virtually
A Medical Device Coordination Group meeting, parts of which are postponed, comes as Germany, home to four of 11 notified bodies designated under EU MDR, anticipates widespread COVID-19 transmission.
By Nick Paul Taylor , Maria Rachal • March 11, 2020 -
FDA updates guidance on 510(k) submissions for electrosurgical devices
The agency offered greater detail on testing requirements in assessing thermal tissue damage.
By Susan Kelly • March 10, 2020 -
ECRI: Poor sterilization, failure to learn from device problems threaten patient safety
The 2020 list put together by the nonprofit ECRI Institute also highlights diagnostic errors as a top patient safety concern for a third consecutive year.
By Maria Rachal • March 10, 2020 -
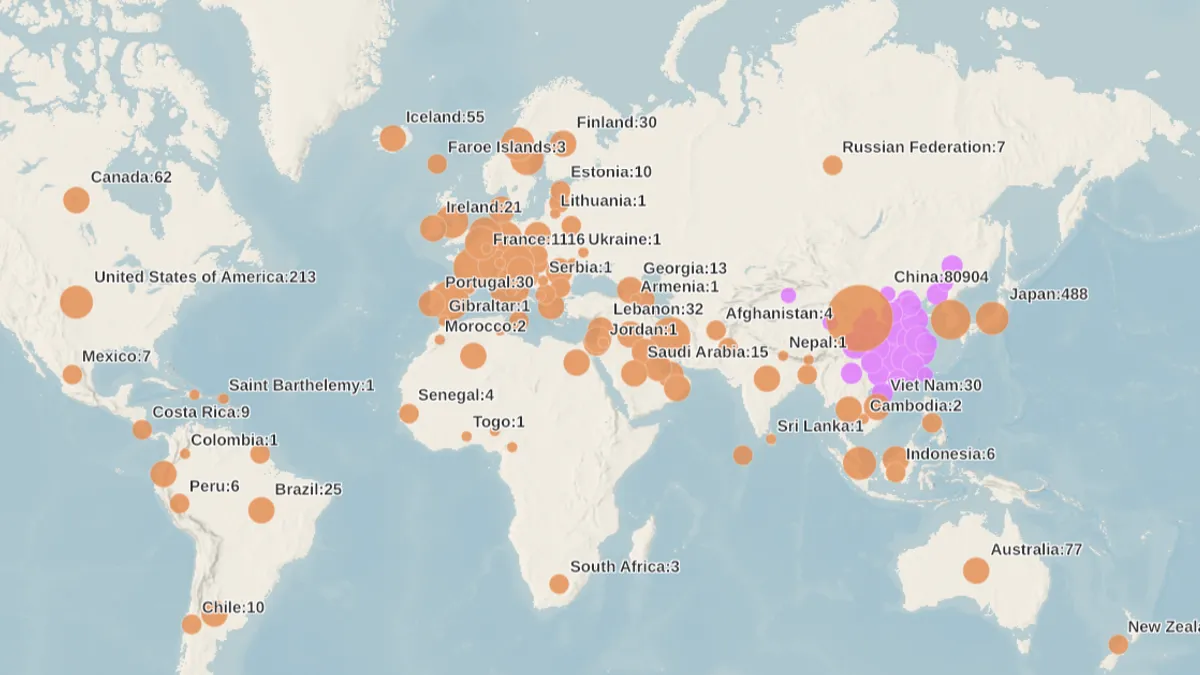
 World Health Organization. (2020). "Map of impact" [Photo]. Retrieved from https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd.
World Health Organization. (2020). "Map of impact" [Photo]. Retrieved from https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd.
Hospitals dipping into emergency stocks to combat coronavirus
As more community spread of COVID-19 is reported daily, hospitals are bracing for a rush of patients and payers are waiving fees for telemedicine consults and extra prescription drug supplies.
By Shannon Muchmore • March 10, 2020 -
Insightec nets up to $150M for incisionless brain surgery tech
Backed by Koch Industries, the privately held medtech makes non-invasive technology that uses ultrasound to help treat ailments such as essential tremor and Parkinson’s disease.
By Greg Slabodkin • March 9, 2020 -
TMS pioneer Neuronetics gets breakthrough nod, CEO departs
The struggling neuromodulation device maker said it will get an expedited FDA review in pursuit of an additional indication for its transcranial magnetic stimulation system in people with bipolar depression.
By Susan Kelly • March 9, 2020 -
FDA gives Class I label to BD Alaris infusion pump system recall
The regulatory agency reported 55 injuries and one death, with 774,000 devices impacted in U.S.
By Greg Slabodkin • March 6, 2020 -
Cardiologist groups back Abbott push to change heart device coverage
Some feedback to CMS supports a retreat from potentially restrictive terms used with ventricular assist devices. Responses to SynCardia’s separate bid to permit the use of artificial hearts outside of clinical trials were more mixed.
By Nick Paul Taylor • March 6, 2020 -
 CDC/Alissa Eckert, MS. "covid-19 coronavirus on black background". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
CDC/Alissa Eckert, MS. "covid-19 coronavirus on black background". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
$8.3B in coronavirus funding set in motion as federal agencies ramp up response
The House and Senate passed a bill with allocations for medical supplies and testing as Vice President Mike Pence met with clinical lab execs Wednesday from LabCorp, Quest Diagnostics, Thermo Fisher and others.
By Shannon Muchmore • Updated March 5, 2020 -
Axonics execs bullish on breaking Medtronic market stranglehold
The medtech contends its rechargeable sacral neuromodulation device is making quicker headway in the marketplace than anticipated as it takes on the incumbent.
By Nick Paul Taylor • March 5, 2020 -
UK looks to use Brexit to make device industry more transparent
The House of Commons bill seeks to address a regulatory gap for medical devices, as the previous legal framework is based on EU directives. One element provides new information-sharing powers related to safety.
By Nick Paul Taylor • March 5, 2020 -
Some pacemakers, EKGs, diabetes devices may face newly flagged Bluetooth cyber risk
FDA and the Department of Homeland Security said microchips from seven manufacturers and a range of consumer wearables and connected medical devices may be affected.
By Susan Kelly • March 4, 2020 -
FDA bans certain electrical stimulation devices called 'barbaric'
Amid mounting pressure from lawmakers, the agency handed down its third-ever medical device ban, finalizing a 2016 proposal prohibiting current and future sale of certain devices to treat self-injury or aggressive behavior.
By Maria Rachal • March 4, 2020 -
Element Science raises $146M to fund rival to Zoll's wearable LifeVest
Deerfield Healthcare and Qiming Venture Partners USA led the round, joining investors Third Rock Ventures and Google Ventures to bring to market Element's wearable defibrillator.
By Nick Paul Taylor • March 4, 2020 -
Hospitals face worries of supply, staff shortages as coronavirus cases tick up
FDA granted a CDC request late Monday for an emergency use authorization that will allow respirators typically used in industrial settings, including N95 masks, to be used in healthcare facilities during the outbreak.
By Shannon Muchmore • March 3, 2020 -
AdvaMed, Medtronic among top 20 pharma and health product lobbyists over 2 decades: study
The medical devices trade association spent $79.4 million on lobbying from 1999 to 2018, making it the fifteenth biggest spender.
By Nick Paul Taylor • March 3, 2020 -
Olympus-backed Medi-Tate gets FDA nod for prostate device
The Japanese corporation has an option to buy the Israeli startup and its newly De Novo-authorized benign prostatic hyperplasia device.
By Nick Paul Taylor • March 3, 2020 -
Supreme Court to again decide fate of ACA
The high court said Monday it would hear the case that seeks to overturn the Affordable Care Act pushed by Texas and other red-leaning states.
By Samantha Liss • Updated March 2, 2020 -
Insulet pauses automated dosing system trial amid software glitch
The delay will likely push back a U.S. product launch of the diabetes technology until early 2021. Also Monday, competitor Tandem said it won its second FDA designation for an interoperable automated glycemic controller.
By Maria Rachal • March 2, 2020 -
Coronavirus: No US shortages of essential devices yet, as FDA monitors more than 60 companies
Still, manufacturers operating 72 plants in China face workforce pressures and surging demand for some equipment, FDA said in an update late Thursday.
By Nick Paul Taylor • Feb. 28, 2020