Medical Devices: Page 115
-
Baxter, B. Braun infusion pumps among millions of devices implicated in Ripple20 cyber alert
Despite warnings that a remote hacker could gain control of a pump, medtechs affected by the vulnerabilities described it as low risk and manageable.
By Greg Slabodkin • June 24, 2020 -
Medtronic Evolut TAVR system gets expanded label in Europe
New indications allow a less-invasive alternative to traditional open heart surgery for a group of severe aortic stenosis patients who tend to be younger and more active, and in higher-risk patients with bicuspid aortic valves.
By Susan Kelly • June 23, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
FDA affirms potential to miss MDUFA deadlines, weighs virtual advisory panels
Separately, the agency announced virtual meetings in September on using patient preference information in medical device regulatory decisions and considering patient-reported outcomes in evaluating devices.
By Nick Paul Taylor • June 23, 2020 -
Mainstay wins FDA approval for chronic back pain device following mixed clinical data
The premarket approval comes 19 months after the ReActiv8 neurostimulation system failed a clinical trial aimed at generating data to break into the U.S. market.
By Nick Paul Taylor • June 23, 2020 -
Moody's: US healthcare system rebounds from COVID-19 in May, but uneven road lies ahead
Last month saw improvement in care volumes, but recent pullbacks in hot spots like Arizona may exemplify the bumpiness to come for recoveries at medical device companies.
By Ron Shinkman • June 19, 2020 -

 Dufour, Tia. (2020). "White House Press Briefing" [Photograph]. Retrieved from Flickr.
Dufour, Tia. (2020). "White House Press Briefing" [Photograph]. Retrieved from Flickr.
Pandemic pushes FDA to 'accelerate' real-world evidence efforts, Hahn says
The agency has grappled with how to leverage real-world data in regulating medical devices, and is now leaning on it to update emergency use authorizations, FDA chief Stephen Hahn said at a forum on Thursday.
By Maria Rachal • June 19, 2020 -
Lack of data encryption for Baxter devices flagged in flurry of DHS alerts
The Department of Homeland Security's cyber agency published four advisories, ranging from 7.5 to 8.6 on its 10-point severity scale, about vulnerabilities in infusion pumps, hemodialysis delivery systems and other tech.
By Nick Paul Taylor • June 19, 2020 -
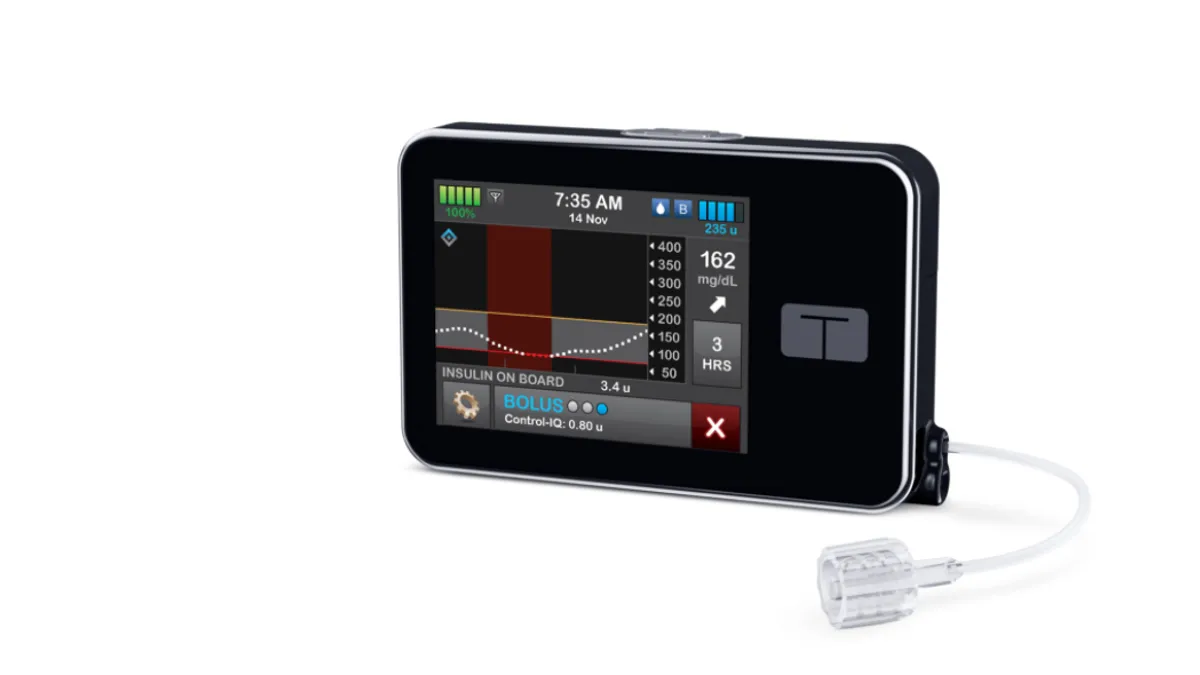
Tandem's insulin dosing algorithm OK'd for use in younger kids
FDA's decision to expand the pediatric indication was supported by an NIH-funded trial that found the automated insulin dosing system helped improve time in range for children ages 6 to 13.
By Maria Rachal • June 17, 2020 -
Thermo Fisher, MedRhythms gain breakthrough device designations
A companion diagnostic for certain brain tumor patients and a digital therapeutic for stroke patients with walking impairments are among the latest technologies tapped by FDA for the priority review program.
By Susan Kelly • June 17, 2020 -
Endologix stent graft tied to 5 deaths gets high-risk recall label from FDA
The Class I recall marks an escalation of a polymer leak issue first reported in 2018. Endologix now says the leaks are due to a "material weakness," not incorrect use as it originally concluded.
By Nick Paul Taylor • June 17, 2020 -
Heart rhythm bodies see 'clear concerns' with using wearables to detect arrhythmias
A paper published on Monday by the Heart Rhythm Society, along with counterpart groups in Asia, Europe and Latin America, found wearable-triggered false positives can cause unwarranted concerns and screening.
By Nick Paul Taylor • June 16, 2020 -
Medtronic inks up to $337M in diabetes funding, Blackstone Life Sciences' 1st medtech investment
The announcement came in conjunction with the ADA's Scientific Sessions this weekend, which also featured data on its advanced hybrid closed loop system that will back an FDA premarket approval submission.
By Susan Kelly • June 15, 2020 -
Paclitaxel-coated balloon, stent labels in Europe to have mortality warning update
A notice posted by British medical device regulators outlined 12 affected products including Boston Scientific's ELUVIA stent, Cook Medical's Zilver PTX stent, and Medtronic's IN.PACT Admiral balloon.
By Greg Slabodkin • June 15, 2020 -
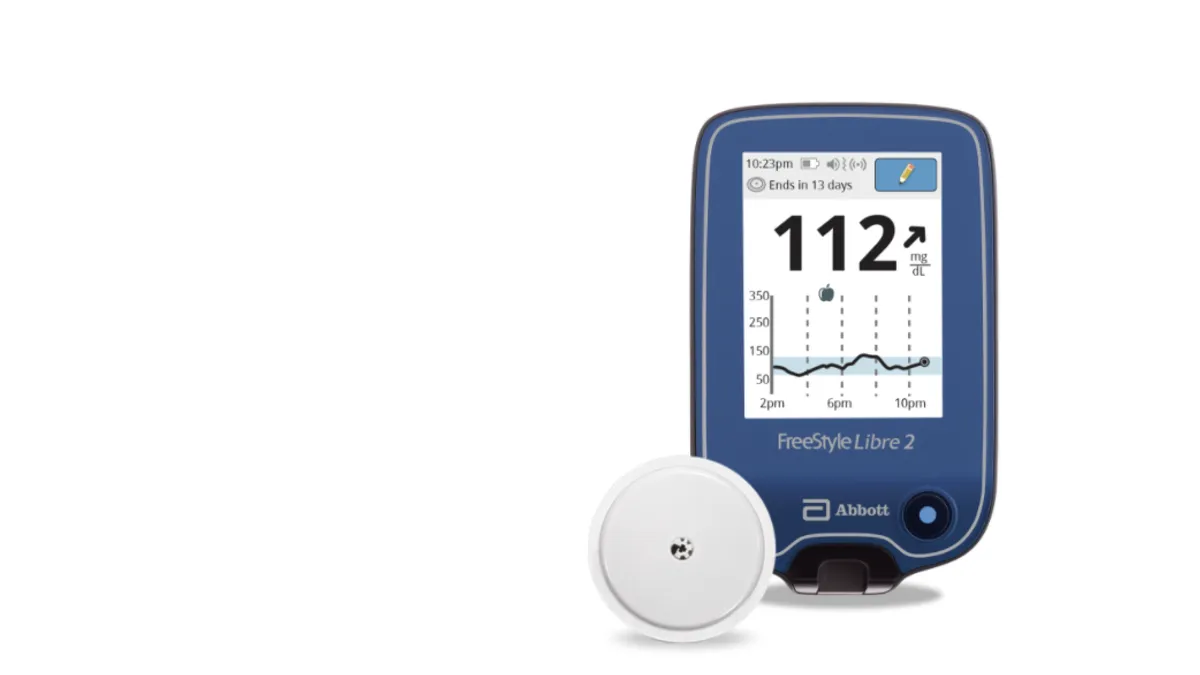
 Retrieved from Abbott/PRNewswire on June 15, 2020
Retrieved from Abbott/PRNewswire on June 15, 2020
Abbott's FreeStyle Libre 2 gets long-awaited iCGM nod from FDA
The designation may stiffen competition for Dexcom, whose G6 device was the sole integrated continuous glucose monitor on the U.S. market. But Abbott's device isn't yet OK'd for use with automated insulin dosing systems.
By Nick Paul Taylor , Maria Rachal • June 15, 2020 -
Key data on Medtronic's 780G, Insulet's Horizon kick off ADA 2020
Abbott, Dexcom and Tandem will also present results at the virtual American Diabetes Association meeting that runs through Tuesday.
By Maria Rachal • June 12, 2020 -
CMS to review at-home ventilation of COPD patients amid variability in device use
Findings from a July Medicare Evidence Development & Coverage Advisory Committee meeting and resulting policy changes could potentially impact ResMed and Philips Respironics.
By Nick Paul Taylor • June 12, 2020 -
Moody's lowers medtech earnings forecast, pegs J&J, Zimmer among hardest hit by COVID-19 fallout
The ratings agency expects the pandemic to curb sales of the large, mostly investment-grade medtechs it covers by 10% this year, with earnings dropping as much as 30%.
By Nick Paul Taylor • Updated June 15, 2020 -
Surgeons, device makers grow more confident in elective care comeback
As some medtechs indicate forecasts entering Q2 may have been too conservative, a Bain & Co. survey of physicians and administrators suggests rising capacity for elective procedures, albeit without sales reps at full force.
By Maria Rachal • June 11, 2020 -
MedTech Europe pitches virtual audits to clear MDR coronavirus logjam
The trade group contends technology such as smart glasses and webcams can enable notified bodies to view facilities without having to visit in person.
By Nick Paul Taylor • June 11, 2020 -

 Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
Trump admin encourages return to elective care, with precautions
Major surgeries should still be limited as much as clinically possible among higher risk patients, and facilities should prepare to screen all visitors and staff for symptoms, according to new CMS guidelines.
By Shannon Muchmore • June 10, 2020 -
Siemens Healthineers, Geisinger ink 10-year digital health partnership
It's one of the medtech's largest partnerships of its kind in North America, as it plans to provide diagnostic imaging and artificial intelligence-enabled applications to the health system over the next decade.
By Greg Slabodkin • June 9, 2020 -
Edwards wins approval to introduce Sapien 3 valve in China
The transcatheter heart device specialist has been eyeing the wealthy, aging nation's untapped market potential for new cardiovascular disease treatments.
By Susan Kelly • June 9, 2020 -
Boston Scientific secures CMS pass-through nod for single-use endoscopes
The alternative payment pathway gives Medicare beneficiaries access to new devices while the agency is still gathering cost data. The company's Exalt D device is the first single-use, flexible duodenoscope to be cleared by FDA.
By Susan Kelly • June 8, 2020 -
Surmodics gets CE mark for Abbott-backed paclitaxel-coated balloon
The company will receive a $10.8 million milestone payment from Abbott, which licensed exclusive commercialization rights to the device for peripheral artery disease patients in 2018 in a bid to take on Medtronic's drug-coated balloons.
By Maria Rachal • June 8, 2020 -
Insulet's pivotal study back on track, setting up Horizon launch in early 2021
The diabetes device maker fixed a software glitch it found with the smartphone-controlled automated insulin delivery system three months ago. Abbott and Dexcom plan to make their CGM tech compatible with the system.
By Maria Rachal • June 5, 2020