Medical Devices: Page 113
-
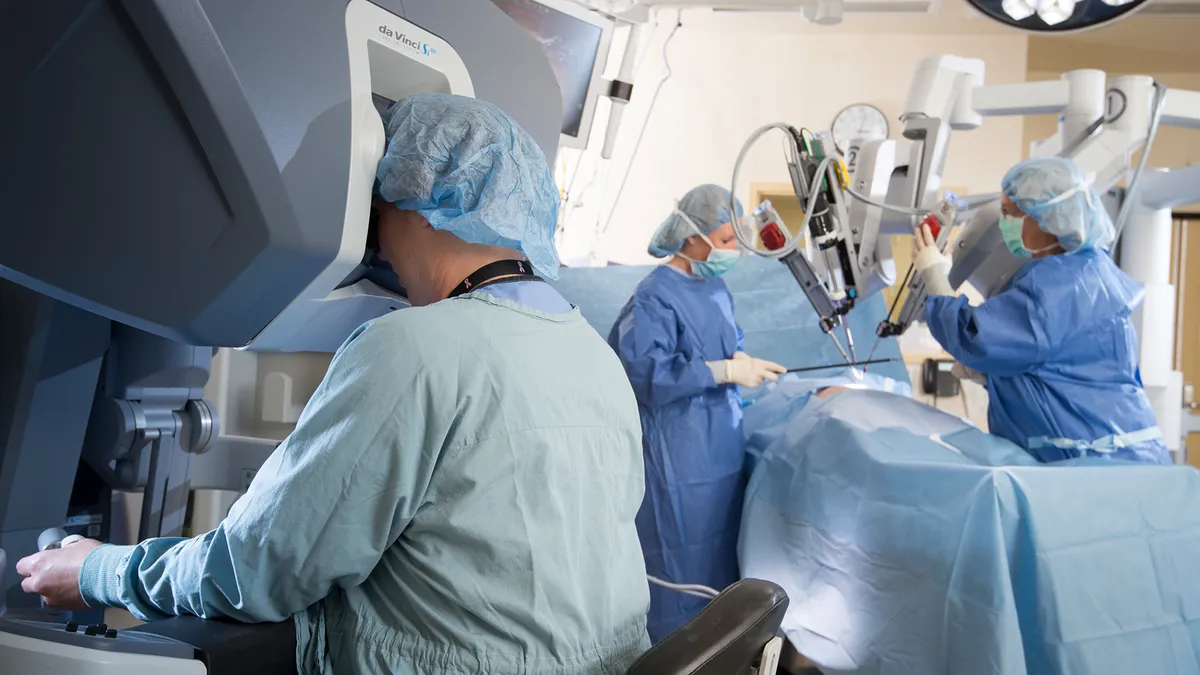
Robot redux: Pandemic scrambles competitive timelines for Intuitive, J&J, Medtronic
Surgeons expect a gradual recovery of robot-assisted procedure volumes in the back half of the year, but the outlook for new robot system placements in hospitals is less optimistic due to freezes in capital equipment budgets.
By Susan Kelly • July 28, 2020 -
Medtech and COVID-19: 6 months into the public health emergency
MedTech Dive examines where the industry stands with testing and the future of those emergency use-authorized diagnostics, as well as how it's adapting to a rebound in elective surgeries and various supply chain challenges.
July 27, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Deep Dive
Medtechs reboot with patients, providers after elective surgeries bottom out
Recovery of procedures critical to device companies came quicker than many in the industry expected. Now, device makers are adapting as business volumes normalize and COVID-19 continues to spread.
By Maria Rachal • July 27, 2020 -
How COVID-19 is challenging and changing medtech supply chain management
Four experts share insights on the supply and demand hangups medtechs have been working through during the pandemic, and a few early takeaways the industry can carry forward.
By Maria Rachal • July 27, 2020 -
COVID-19 diagnostics emerging as prime investment target, SVB analysis shows
Companies with tech involved in the pandemic response raised $1.3 billion over the first half of the year, with developers of tests and R&D tools enjoying a 62% increase in investment.
By Nick Paul Taylor • July 24, 2020 -
Edwards aims for 5% TAVR sales growth in 2020 after seeing demand bounce back
Quarterly sales came in $25 million above the top end of the device maker's guidance, with 90% of its active sites having done TAVR procedures in May and June. Yet, there are concerns regarding a slowdown in new patient screenings.
By Nick Paul Taylor • July 24, 2020 -
HHS renews COVID-19 public health emergency
The extension supported by AdvaMed, the American Clinical Laboratory Association and American Hospital Association takes effect Saturday and opens the door for FDA to keep issuing emergency use authorizations.
By Shannon Muchmore • July 23, 2020 -
Medical device deal values down 88% amid COVID-19 disruption: PwC
Going forward, M&A may rise in light of recent deferral of elective procedures, which analysts say “could create the need for significant consolidation as companies work to remain competitive.”
By Nick Paul Taylor • July 23, 2020 -
Boston Scientific to begin limited US launch of latest Watchman device
The medtech hopes the updated version of its high-growth stroke technology will help boost its interventional cardiology unit as elective procedures pick back up.
By Maria Rachal • July 22, 2020 -
Intuitive plans to lower instrument prices as it warns of long recovery
The surgical robots maker reported a pullback in revenue and earnings as it weathers the one-two COVID-19 punch of delayed elective procedures and constrained capital equipment spending.
By Susan Kelly • July 22, 2020 -

 Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
AdvaMed launches COVID-19 test registry to address shortages
Created in partnership with Abbott, Roche, Siemens and others, the database is aimed at helping ensure sufficient testing supply by providing weekly state- and national-level updates on the number of tests shipped.
By Nick Paul Taylor • July 22, 2020 -
NuVasive surprises analysts with sooner-than-expected spine surgery sales rebound
Preliminary second quarter results show sales were down 30% year over year but were about a third higher than average analyst expectations, adding to evidence of a faster-than-predicted COVID-19 recovery in the medtech sector.
By Nick Paul Taylor • July 21, 2020 -
As pandemic rages on, hip and knee surgeons lay out elective procedure guidance
An international group of orthopaedic experts outlined recommended changes to hospital protocols to prevent transmission of COVID-19 when elective procedures resume.
By Susan Kelly • July 20, 2020 -
Philips sees double-digit COVID-19 connected care bump amid Q2 sales dip
Headwinds won out this spring, dragging total sales down 6%. Still, management predicted the return of elective procedures will help tip the balance to growth for the rest of the year.
By Nick Paul Taylor • July 20, 2020 -
Ambu wins FDA clearance for single-use duodenoscope product
Boston Scientific was first to obtain an agency nod for a fully disposable version, meant to be safer against infection, which it believes could be worth more than $1 billion.
By Maria Rachal • July 17, 2020 -
J&J drops planned 510(k) for Verb-Auris robot, targets 2022 clinical trial start
Talks with the FDA led Johnson & Johnson to opt against trying to bring its general surgery robotics platform to market via the 510(k) pathway. Launch delays buy market incumbent Intuitive some important time, analysts noted.
By Nick Paul Taylor • July 17, 2020 -
J&J device sales fell by one-third in Q2, but June rebound drives better 2020 outlook
Device sales in some areas returned to around 90% of pre-pandemic levels by the end of the quarter, causing J&J to improve its 2020 worst-case scenario for the division by $1.75 billion.
By Nick Paul Taylor • July 16, 2020 -
Abbott tops Q2 estimates aided by 233.6% jump in molecular diagnostics
The medtech giant reported $7.3 billion in second quarter revenue, including $615 million from coronavirus testing products. Molecular diagnostics sales were driven by strong demand for its point-of-care ID NOW platform.
By Greg Slabodkin • July 16, 2020 -
Medtronic closes Medicrea buyout, seeking spinal surgery boost
Four months after announcing the deal for the French medtech, Medtronic also highlighted its expanding foothold in AI, machine learning and predictive analytics.
By Maria Rachal • Updated Nov. 16, 2020 -
UK signals Stryker offer to unload ankle replacement product could clear Wright Medical buy
The medtech giant's proposed $4 billion acquisition prompted competition concerns among regulators. Stryker disclosed in a filing Wednesday it pitched divesting its STAR product line, which may satisfy U.K. authorities.
By Greg Slabodkin • July 15, 2020 -
FDA commits to staggered move to electronic medtech filings
Finalized guidance on electronic submissions of medical device regulatory filings takes into account AdvaMed comments on timing.
By Nick Paul Taylor • July 15, 2020 -
Smith & Nephew launches new hand-held robotic tool amid COVID-19 health sector pullback
The London-based medtech is betting portability and potentially faster knee procedure times will attract customers despite a slowdown in capital equipment orders and drop in elective surgeries during the pandemic.
By Susan Kelly • July 15, 2020 -
ElectroCore noninvasive nerve stimulator gets EUA for COVID-19 patients with asthma
The small medtech has focused on migraine and cluster headache, but FDA's support could be a first step to developing evidence to back expansion of noninvasive vagus nerve stimulation into reactive airway disease.
By Susan Kelly • July 14, 2020 -
FDA proposes BPH device trial guidance changes as market heats up
The updates come as a swath of companies take aim at the benign prostatic hyperplasia opportunity, which medtechs like Boston Scientific and Teleflex have invested in more since FDA issued development guidance a decade ago.
By Nick Paul Taylor • July 14, 2020 -
Edwards to pay Abbott $468M to settle mitral, tricuspid repair device patent disputes
Jefferies analysts called the settlement "a very good deal" for Edwards as it opens up a market opportunity that may be worth $3 billion come 2024.
By Nick Paul Taylor • July 14, 2020