Medical Devices: Page 107
-
Medtronic to pay $9.2M to settle DOJ influence, kickback allegations
The medtech giant is accused of offering "lavish" benefits in promoting a certain infusion pump to a South Dakota neurosurgeon, and misreporting those payments under a required CMS program.
By Nick Paul Taylor • Oct. 30, 2020 -
Medtronic acquires thyroid surgery tech in 7th tuck-in deal of 2020
An addition to the medtech's pandemic-battered ENT business, Ai Biomed's PTeye system won De Novo authorization from FDA in 2018 for its ability to help surgeons spare important calcium-regulating tissue during procedures.
By Maria Rachal • Oct. 29, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
Baxter Q3 sales up 4% as virus-driven demand offsets headwinds
The renal care business continues to grow during the pandemic but lower ER utilization and fewer hospitalizations, particularly in the U.S., hurt revenues by more than $65 million in the quarter.
By Greg Slabodkin • Oct. 29, 2020 -
Teleflex lines up $525M to buy hemostat specialist Z-Medica
The medtech predicts the trauma surgery and emergency medicine-focused products will begin adding up to $70 million in revenue next year, providing growth drivers outside its key interventional urology segment.
By Maria Rachal • Oct. 29, 2020 -
CMS competitive bidding process fails to drive expected savings, sparking rethink
While Needham analysts contend one action removes an overhang for Inogen, Invacare and ResMed, those at Jefferies see a "major negative" in the agency's determination to lower rates outlined in a proposed rule.
By Nick Paul Taylor • Oct. 29, 2020 -
Smith & Nephew lays path to 10% growth for acquired Integra assets
The medtech is moving deeper into the extremity orthopaedics turf fought over by the likes of Zimmer Biomet and Stryker acquisition target Wright Medical.
By Nick Paul Taylor • Oct. 29, 2020 -
Dexcom revenue growth dampened amid shift to lower pharmacy prices
The 26% rise in Q3 revenue, which came from a nearly 40% increase in sales, beat analysts' consensus estimates and were good enough for the company to raise its 2020 outlook. Still, shares closed down 9% on Tuesday.
By Maria Rachal • Oct. 28, 2020 -
Neovasc angina device fails to win FDA panel backing, stock tumbles 43%
By a vote of 17-1, experts on FDA's circulatory system devices panel said the available data did not provide reasonable assurance of effectiveness, though most thought the Reducer system was safe for patients.
By Susan Kelly • Oct. 28, 2020 -
Boston Scientific sales not yet back to growth as Watchman hit compounds COVID-19 impacts
The medtech is aiming for organic revenue growth in Q4 after falling 5.7% in Q3. CEO Mike Mahoney said estimating the remaining procedure backlog and new patient funnel "remain a challenge" and vary by business and region.
By Nick Paul Taylor • Oct. 28, 2020 -
MDUFA V talks kick off as FDA grapples with onslaught of COVID-19 submissions
Medtech industry groups broadly expressed a desire to maintain the status quo after FDA Commissioner Stephen Hahn described the strain on agency workers under MDUFA IV as unsustainable.
By Maria Rachal • Oct. 28, 2020 -
Neovasc refractory angina treatment faces FDA panel review
The agency's ultimate approval decision on the Canadian company's CE-marked device has important financial implications for the medtech, which saw its revenue plunge and operating loss deepen in the second quarter.
By Susan Kelly • Oct. 27, 2020 -
 Retrieved from Abiomed/BusinessWire on April 30, 2020
Retrieved from Abiomed/BusinessWire on April 30, 2020
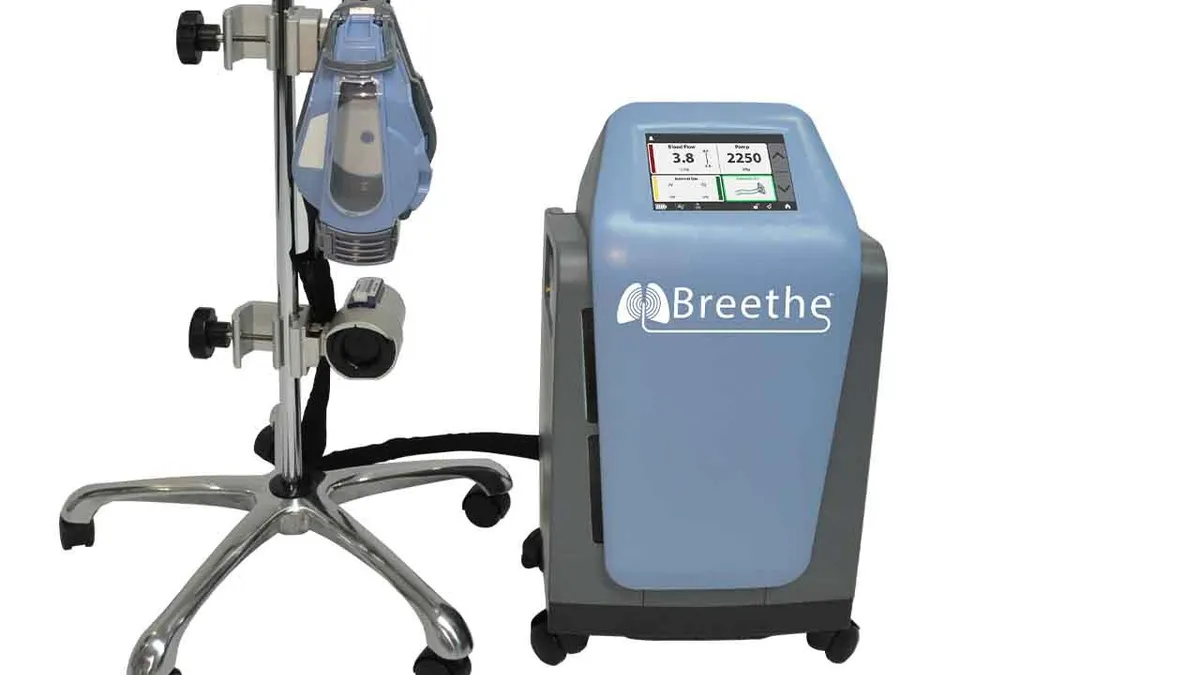
FDA grants 510(k) to Abiomed's artificial lung, teeing up use in COVID-19
While LivaNova and Medtronic already sell ECMO devices, Abiomed is focusing on a relatively compact design to differentiate the device from the pack.
By Nick Paul Taylor • Oct. 27, 2020 -
 Retrieved from Pixabay.
Retrieved from Pixabay.
Despite recent rebound, hospitals find admissions still well below pre-pandemic levels
Trends from health systems in New York and California suggest patients may have lost health insurance as a result of the economic fallout from the pandemic
By Ron Shinkman • Oct. 26, 2020 -
FDA, Philips warn of data bias in AI, machine learning devices
The comments come 18 months after FDA unveiled a yet-to-be-finalized framework for modifying AI/ML-based software as a medical device using real-world learning and adaptation.
By Greg Slabodkin • Oct. 26, 2020 -
Stryker buyout of Wright poised to pass 1-year mark without closing
CEO Kevin Lobo said last month the $5.4 billion buyout, which has faced antitrust hurdles, was on track to be finalized in early October.
By Maria Rachal • Oct. 26, 2020 -
EU notified body designation pipeline points to IVDR bottleneck
An update from the European Commission reveals only a few notified bodies are likely to join the four already designated over the coming months.
By Nick Paul Taylor • Oct. 26, 2020 -
Medtech Q3 results signal diagnostics boom, mixed bag on device growth
Hologic, BD and Zimmer Biomet are among the medtechs set to add to the growing mass of late summer data points this week.
Updated Nov. 2, 2020 -
Neuromod devices at the fore in latest FDA breakthrough designations
Liquid biopsies also stand out as an area where U.S. regulators are encouraging development and prioritizing review.
By Nick Paul Taylor • Oct. 23, 2020 -
Robotic surgery pricier, with no measurable benefit in hernias: Cleveland Clinic trial
Certain procedures with Intuitive's da Vinci took 55% longer than those done laparoscopically, which drove up costs. The findings also punctured the perception that robotic platforms may reduce postoperative pain.
By Nick Paul Taylor • Oct. 22, 2020 -
Edwards TAVR sales grow 6% but Q4 forecast disappoints
A resurgence of COVID-19 cases in Europe and parts of the U.S. creates uncertainty about the fourth quarter, execs acknowledged, but noted talks with hospitals suggest widespread global disruption to surgeries is unlikely.
By Nick Paul Taylor • Oct. 22, 2020 -
Abbott device growth returns as Wall Street queries staying power of COVID-19 test boom
CEO Robert Ford argued that focusing on how coronavirus test demand will evolve "misses the point," touting the longer-term benefits of its now-expanded diagnostic platforms footprint.
By Maria Rachal • Oct. 21, 2020 -
FDA floats framework to message cybersecurity threats to patients
The proposal, which has the agency and industry sharing responsibility for making the information easy to find, comes as the risks to medical devices continue to grow.
By Nick Paul Taylor • Oct. 21, 2020 -
Robotic surgery startups help drive Q3 medical device funding over $5B, an all-time high
CB Insights also listed the progress of neuromodulation devices and Medtronic's deals in diabetes and neurosurgery as other highlights.
By Nick Paul Taylor • Oct. 20, 2020 -
FDA, medtechs envision new era for clinical trials amid COVID-19 shake-up
Medtronic, for example, has faced "very significant delays" in getting products approved as a result of research disruptions, the company's VP of global clinical affairs said during a discussion at TCT.
By Maria Rachal • Updated Oct. 20, 2020 -
Future of COVID-19 products, device shortages, CDS top CDRH 2021 to-do list
Regulators aim to publish final guidance on five topics and draft documents on another dozen priority areas in the coming year. A few of the goals are carryovers as priorities shifted during the public health emergency.
By Susan Kelly • Oct. 19, 2020