Medical Devices
-
Bausch + Lomb recalls Envista intraocular lenses over safety risks
J.P. Morgan analysts said it was too early to discuss the financial impact of the recall, but estimated that $70 million to $90 million of revenue could be at risk this year.
By Nick Paul Taylor • March 31, 2025 -
Tandem eyes Medicare coverage expansion for Type 2
CMO Jordan Pinsker said Tandem hopes to expand Medicare coverage of its automated insulin delivery systems using new data shared at ATTD.
By Elise Reuter • March 31, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
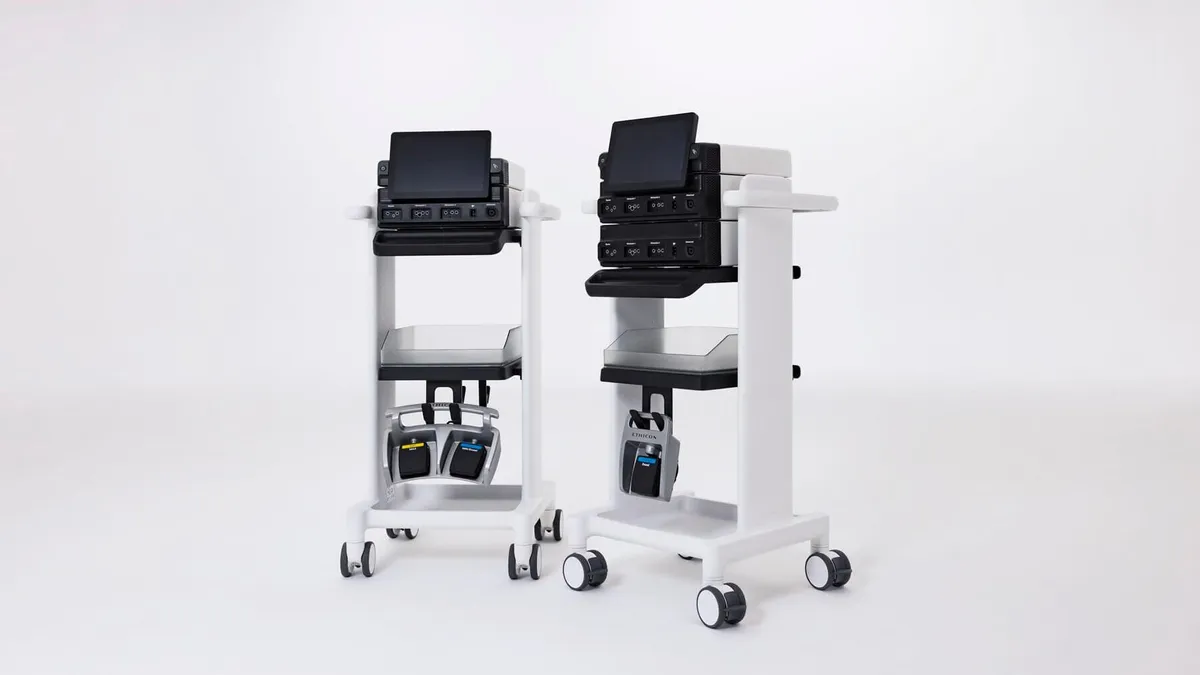
J&J launches energy generator compatible with Ottava robot
Johnson & Johnson said the Dualto energy system’s design allows for up to 46% less footprint than separate generators.
By Nick Paul Taylor • March 28, 2025 -
‘Everything is word of mouth’: HHS employees face uncertainty in looming Trump layoffs
Many HHS department heads and employees were unaware of plans to fire some 10,000 employees until they were announced Thursday, sources said.
By Sydney Halleman , Ned Pagliarulo , Rebecca Pifer • March 28, 2025 -
Abbott snags early CE mark for Volt PFA device
With PFA becoming physicians’ preferred ablation method for treating AFib, Abbott is pushing to catch up with rival systems already on the market.
By Susan Kelly • March 28, 2025 -
Newly public Beta Bionics reports sales surge; Dexcom hires CCO
Sales of Beta Bionics insulin pumps spiked 145% in the fourth quarter behind solid new patient starts. Elsewhere, former Masimo exec Jon Coleman joined Dexcom, and Sibionics’ CGM earned the CE mark.
By Nick Paul Taylor • March 28, 2025 -
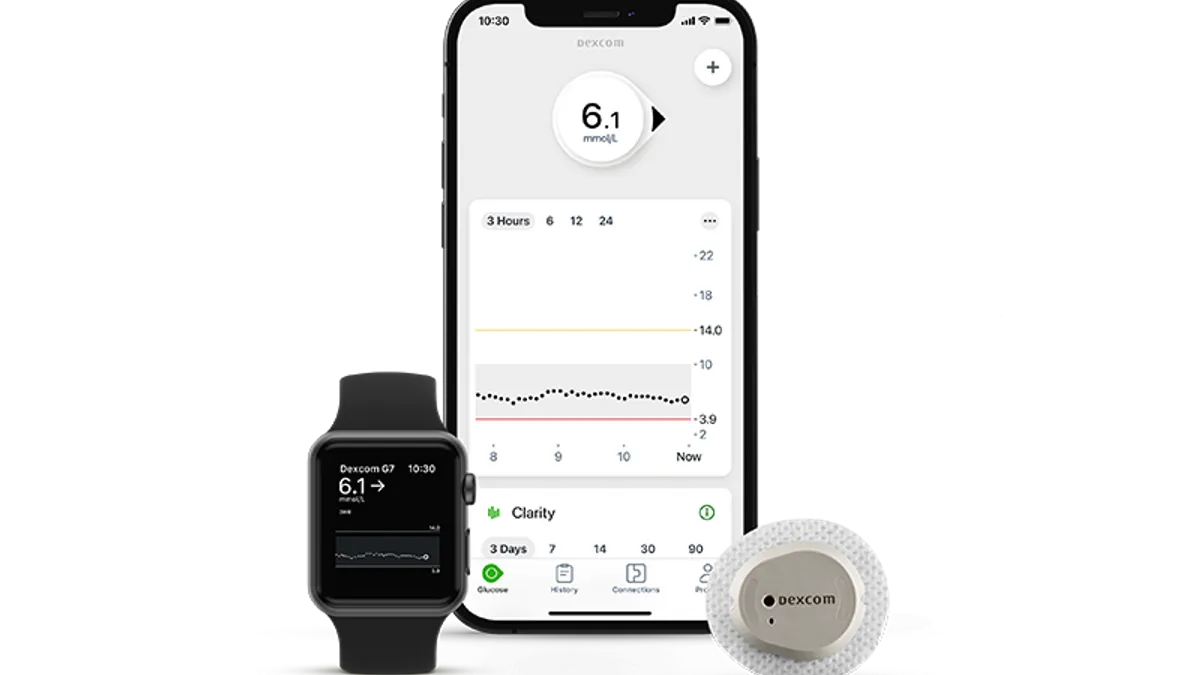
Dexcom rejects claims of unauthorized device changes in FDA warning letter
A company spokesperson said Dexcom plans to resolve the agency's concerns but stated no design changes were made to its glucose sensors.
By Elise Reuter • March 27, 2025 -
HHS to cut 10,000 employees in major restructuring under RFK Jr.
Advamed CEO Scott Whitaker said Thursday that “any reduction in force should be accompanied by policy and regulatory improvements that encourage innovation in medtech.”
By Ned Pagliarulo , Rebecca Pifer , Ricky Zipp • Updated March 27, 2025 -
Solventum cuts 800 positions
CEO Bryan Hanson told investors last week that the restructuring plan would save Solventum $120 million annually.
By Ricky Zipp • March 26, 2025 -
Senate committee advances Dr. Oz to lead CMS
Democrats on the Senate Finance Committee criticized the physician and TV personality ahead of the vote Tuesday for avoiding questions about potential cuts to Medicaid.
By Emily Olsen • March 26, 2025 -
Dexcom’s FDA warning letter reveals unauthorized changes to sensors
Dexcom made a significant design change to a component used in its sensors and did not adequately validate the change, according to the warning letter.
By Elise Reuter • March 26, 2025 -
Q&A
Dexcom COO on 15-day glucose sensor, Type 2 coverage
Jake Leach caught up with MedTech Dive during the ATTD conference to discuss new data on the company’s glucose monitors and an FDA warning letter.
By Elise Reuter • March 25, 2025 -
Deep Dive
5 tips for building a predetermined change control plan
The new framework is intended to make postmarket changes easier for products. Experts recommend having a clear roadmap and contacting regulators early.
By Elise Reuter • March 25, 2025 -
Abbott gets FDA nod to begin IVL trial after rivals buy up competition
J&J acquired the IVL device maker Shockwave Medical last year for $13.1 billion, and Boston Scientific agreed to buy Bolt Medical in January for up to $664 million.
By Nick Paul Taylor • March 25, 2025 -
23andMe files for bankruptcy; CEO Anne Wojcicki resigns
The DNA-testing company plans to sell its assets after the board rejected an acquisition proposal from Wojcicki.
By Elise Reuter • March 24, 2025 -
Q&A
Why HCA is ‘all in’ on surgical robotics
Thomas Payne, HCA Healthcare’s national medical director of robotics, explains how the largest U.S. health system is vetting a new crop of market entrants.
By Susan Kelly • March 24, 2025 -
J&J boosts planned US manufacturing investment to $55B
Following big announcements from Lilly and Merck, J&J is pledging $55 billion over the next four years to open new plants.
By Jonathan Gardner • March 21, 2025 -

 Retrieved from ICU Medical on March 21, 2025
Retrieved from ICU Medical on March 21, 2025
Smiths Medical recalls port implants, warns on endotracheal tubes
The business, acquired by ICU Medical in 2022, has been dealing with a series of recalls and quality issues over the past several years.
By Susan Kelly • March 21, 2025 -
FDA posts early alert after Calyxo urinary stone device tied to death
The alert describes a problem that can lead to excessive pressure in the kidney during procedures to remove urinary stones.
By Nick Paul Taylor • March 21, 2025 -
Tariffs are paused for USMCA-compliant goods. How can companies qualify?
Businesses that previously found pursuing USMCA treatment cost-prohibitive are scrambling to determine if they meet the trade deal’s requirements.
By Philip Neuffer • March 20, 2025 -
Dexcom shares 15-day CGM data; Tandem posts Type 2 pivotal data
Medical device companies presented new data this week at the Advanced Technologies & Treatments for Diabetes conference.
By Elise Reuter • March 20, 2025 -
Medtronic recalls embolization devices tied to 17 injuries, 4 deaths
Medtronic will remove one model of Pipeline Vantage devices from where they’re used and sold and will update the instructions for another model.
By Nick Paul Taylor • March 19, 2025 -
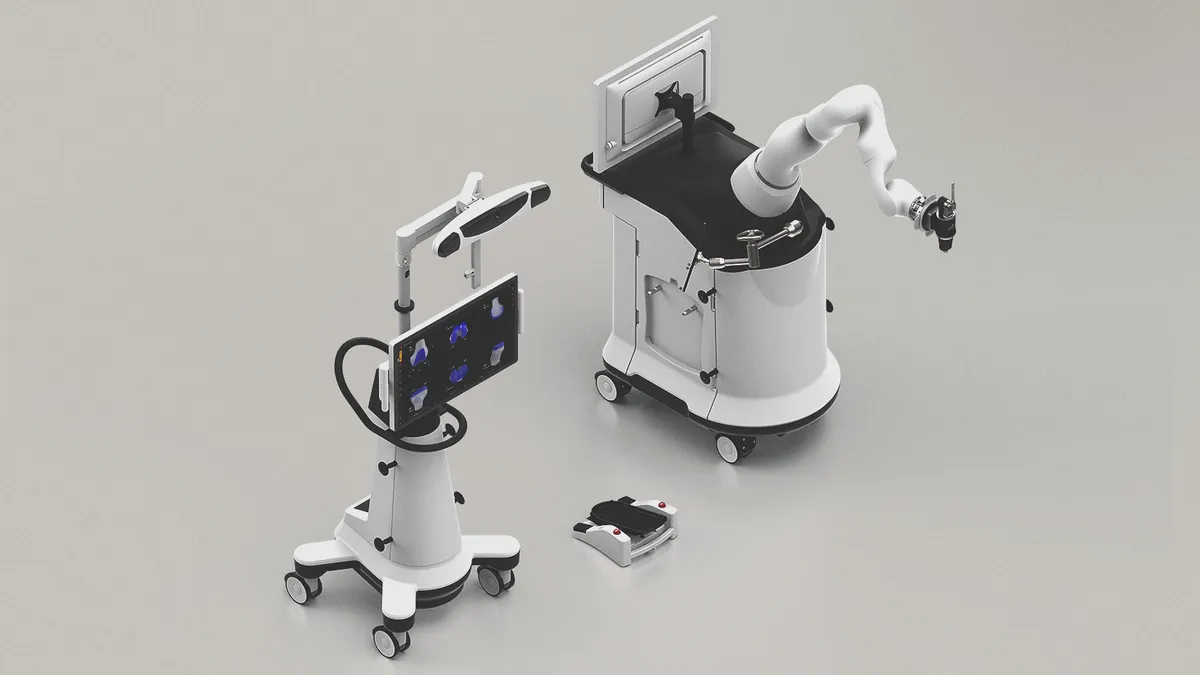
Monogram robot wins FDA OK; Vicarious hit by supplier woes
Monogram Technologies secured 510(k) clearance for its robotic knee replacement system, while Vicarious Surgical blamed supply chain issues for delaying its regulatory timeline.
By Susan Kelly • March 19, 2025 -

 Retrieved from Insulet on March 18, 2025
Retrieved from Insulet on March 18, 2025
Insulet Chief Technology Officer Mark Field leaves
Amit Guliani, group vice president of software engineering, was named acting chief technology officer, effective immediately.
By Elise Reuter • March 18, 2025 -


Courtesy of Nucor Steel Tuscaloosa.

Tariff uncertainty sparks manufacturing anxiety, especially among small firms
Shifting foreign suppliers could become more difficult as the Trump administration continues to enact new widespread tariff policies.
By Kate Magill • March 18, 2025