Medical Devices
-
FDA seeks feedback on monitoring real-world performance of AI devices
The agency is looking for ways to detect, assess and mitigate changes to the performance of AI-enabled devices over time.
By Nick Paul Taylor • Oct. 1, 2025 -
Boston Scientific unveils plans for new Watchman device
The company hopes to launch its next-generation Watchman system in the second half of 2027 or early 2028.
By Elise Reuter • Oct. 1, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Top medtech conferences in 2026
The medical device industry has a jam-packed year of events for professionals to attend, including the J.P. Morgan Healthcare Conference, DeviceTalks Boston and AdvaMed’s annual medtech meeting.
By Ricky Zipp • Oct. 1, 2025 -
FDA expands early alert program to cover all medical devices
After a successful pilot, the agency has lifted the limitations on when it will provide early information about potentially high-risk safety events.
By Nick Paul Taylor • Sept. 30, 2025 -
Medtech firms splitting into ‘haves’ and ‘have-nots’: EY
The number of medtech funding rounds has declined in recent months, but the value of overall deals has increased, according to a new report from EY.
By Elise Reuter • Sept. 29, 2025 -
EssilorLuxottica wins FDA OK for myopia-slowing eyeglass lenses
Investigators saw a 71% reduction in myopia progression in children who used the lenses for two years.
By Nick Paul Taylor • Sept. 29, 2025 -
Biolinq gets FDA de novo nod for intradermal glucose sensor
The device, called Biolinq Shine, is indicated for people with Type 2 diabetes who don’t depend on insulin.
By Elise Reuter • Updated Sept. 29, 2025 -
SS Innovations hires new CFO
Naveen Kumar Amar joins the surgical robot developer as it prepares to expand globally, including in Europe and the U.S.
By Susan Kelly • Sept. 26, 2025 -
MedTech Europe pressures EU to make regulatory changes by early 2026
The request includes a call to postpone re-certification requirements for devices already certified under the medical technology regulations.
By Nick Paul Taylor • Sept. 26, 2025 -
Section 232 probe reignites tariff uncertainty for medtech firms
The Trump administration has used the investigations in other sectors to implement tariffs, but the earliest any potential actions would take place is summer 2026, analysts said.
By Elise Reuter • Sept. 26, 2025 -
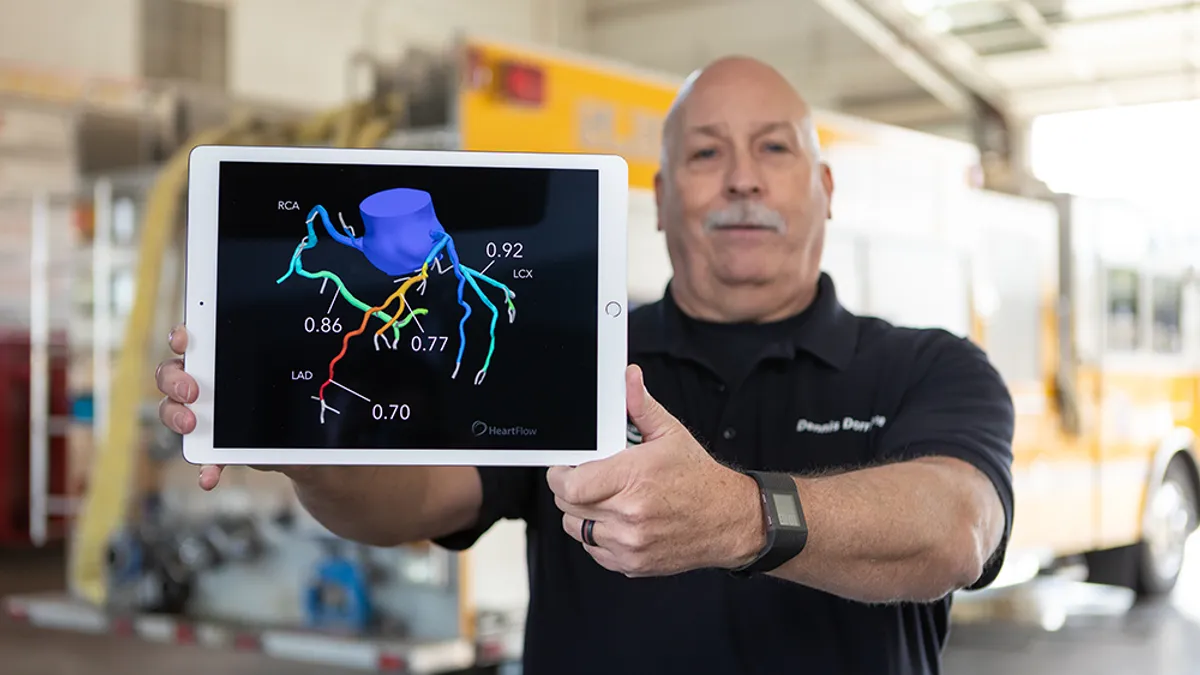
Heartflow receives FDA clearance for updated plaque analysis platform
The updated algorithm shows a 21% improvement in plaque detection, compared to the original version.
By Nick Paul Taylor • Sept. 25, 2025 -
US opens investigation into medical equipment, device imports
As part of the Section 232 probe, the Trump administration will weigh the need for tariffs on goods such as surgical masks, blood glucose monitors and wheelchairs.
By Philip Neuffer • Sept. 25, 2025 -
Q&A
Que Dallara on Medtronic’s diabetes spinoff, pipeline and Abbott partnership
Dallara, president of Medtronic’s diabetes business, discussed the segment’s turnaround and why she sees a benefit in becoming a standalone firm.
By Elise Reuter • Sept. 25, 2025 -
Medtronic expands AI, robotics hub in London; Virtuoso robot gets FDA breakthrough status
The Medtronic center will facilitate surgeon collaboration and AI-powered decision support. At Virtuoso Surgical, a robotic approach to bladder lesion removal is expected to improve precision.
By Susan Kelly • Sept. 24, 2025 -
Cardiosense names Eric Meizlish as CEO
Cardiosense’s switch to a leader with experience scaling healthtech companies comes as it works to build on the recent 510(k) clearance of CardioTag.
By Nick Paul Taylor • Updated Sept. 25, 2025 -
J&J’s Abiomed starts third heart pump controller recall since June
As of Aug. 27, the company had reported five serious injuries and no deaths associated with the issue.
By Nick Paul Taylor • Sept. 24, 2025 -

 Retrieved from Johnson & Johnson on September 23, 2025
Retrieved from Johnson & Johnson on September 23, 2025
J&J exits Linx esophageal reflux business in some countries
Johnson & Johnson, which said it made the decision based on a market assessment, did not specify the markets it is leaving or provide a timeline. Linx will remain available in the U.S.
By Susan Kelly • Updated Sept. 24, 2025 -
Olympus removes needles after patient death
Olympus is recalling certain lots of needles, used for lung cancer biopsy, because components can detach during procedures.
By Elise Reuter • Updated an hour ago -
Medtronic wins FDA approval for urinary incontinence device
Analysts said Altaviva is an attractive option compared to rival products, informing their belief that Medtronic can capitalize on a large market opportunity.
By Nick Paul Taylor • Sept. 22, 2025 -
Profile
How SS Innovations is expanding robotic surgery’s reach
Heart surgeon Sudhir Srivastava saw a global need for less-invasive surgical care at an affordable price. His company, SS Innovations, built a robot that has now been used in more than 5,000 surgeries.
By Susan Kelly • Sept. 22, 2025 -
Exactech agrees to $8M settlement over defective knee implants
The payment resolves allegations brought by the Department of Justice that Exactech violated the False Claims Act by selling knee implants with components that failed prematurely.
By Elise Reuter • Sept. 19, 2025 -
FDA posts early alert about Abbott’s Tactiflex Ablation Catheter
Catheter tips came off three damaged devices during surgery but none of the patients suffered serious injuries or died.
By Nick Paul Taylor • Updated Sept. 22, 2025 -
J&J launches IVL device in Europe; Medtronic looks at pacing for more patients
J&J’s Shockwave Javelin intravascular lithotripsy catheter treats people with peripheral artery disease. Elsewhere, Medtronic began a pivotal trial to evaluate its pacemakers in a new patient group.
By Susan Kelly • Sept. 18, 2025 -
House committee advances Medicare coverage bill for breakthrough devices
AdvaMed welcomed the committee’s work on the legislation but called for the inclusion of diagnostics in the bill.
By Nick Paul Taylor • Sept. 18, 2025 -
FDA warns on unauthorized blood pressure, infant monitoring devices
Many of these devices currently sold over-the-counter do not have marketing authorization, the agency said in a notice.
By Elise Reuter • Updated Sept. 18, 2025