Digital Health: Page 26
-
BARDA backs push for clinical-grade wearable to detect signs of COVID-19
As companies like Fitbit aim to leverage their wrist-worn devices to monitor for disease symptoms, developers at Northwestern University argue that consumer tech falls short compared to clinical-grade, chest and neck-worn wearables.
By Nick Paul Taylor • July 2, 2020 -
Sidekick, Pfizer team on digital therapeutic for chronic disease
The collaboration marks the second partnership with the drug giant in under a year for the 6-year-old digital therapeutics maker, which also is working with Bayer on a behavior modification and medication management tool.
By Susan Kelly • June 30, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Sitthiphong via Getty Images
Sitthiphong via Getty Images Trendline
TrendlineMedical device industry continues to turn to AI
While the industry continues to embrace artificial intelligence, there are still questions about how the new technologies need to be regulated and if they are effective.
By MedTech Dive staff -
AdvaMed, tech groups urge Congress to make eased telehealth rules permanent
Separately, Jefferies analysts pegged Teladoc, Quest Diagnostics, Exact Sciences and CareDx as beneficiaries of the boost in virtual care that's come during the COVID-19 pandemic.
By Greg Slabodkin • June 30, 2020 -
FDA nod to Boston Scientific cardiac monitor clears challenge to Abbott, Medtronic
The medtech giant is betting remote adjustment will put its LUX-Dx insertable system in a position to take on rivals, which have a years-long head start.
By Nick Paul Taylor • June 29, 2020 -
CMS wants to make home health telemedicine permanent after COVID-19
Agencies would be able to keep using the tech for beneficiaries under the Medicare home health benefit beyond the public health emergency, including remote patient monitoring, according to a proposed rule fact sheet.
By Rebecca Pifer • June 26, 2020 -

 "Man in face mask in front of Union Station at 50 Massachusetts Avenue, NE, Washington DC on Thursday morning,19 March 2020" by Elvert Barnes Photography is licensed under CC BY-SA 2.0
"Man in face mask in front of Union Station at 50 Massachusetts Avenue, NE, Washington DC on Thursday morning,19 March 2020" by Elvert Barnes Photography is licensed under CC BY-SA 2.0
BARDA backs study of Empatica's COVID-19 early warning platform
The system, which measures physiological data including blood volume pulse and electrodermal activity, is the latest in a growing list of wearables being primed to detect and monitor infections.
By Susan Kelly • June 22, 2020 -
Lack of data encryption for Baxter devices flagged in flurry of DHS alerts
The Department of Homeland Security's cyber agency published four advisories, ranging from 7.5 to 8.6 on its 10-point severity scale, about vulnerabilities in infusion pumps, hemodialysis delivery systems and other tech.
By Nick Paul Taylor • June 19, 2020 -
Senators back sustaining telehealth momentum post pandemic
Top priorities include axing geographic restrictions, expanding Medicare and Medicaid reimbursement for virtual care services, and upping use of remote patient monitoring and digital health tools for patients with chronic conditions.
By Rebecca Pifer • June 18, 2020 -

 Retrieved from Akili Interactive/Businesswire on June 15, 2020
Retrieved from Akili Interactive/Businesswire on June 15, 2020
In FDA 1st, game-based therapeutic gets marketing OK
Digital therapeutics developer Akili Interactive Labs won De Novo authorization for a treatment meant to improve attention function in children with ADHD, building on a rollout it began under a special COVID-19 policy.
By Maria Rachal • June 16, 2020 -
Heart rhythm bodies see 'clear concerns' with using wearables to detect arrhythmias
A paper published on Monday by the Heart Rhythm Society, along with counterpart groups in Asia, Europe and Latin America, found wearable-triggered false positives can cause unwarranted concerns and screening.
By Nick Paul Taylor • June 16, 2020 -
Siemens Healthineers, Geisinger ink 10-year digital health partnership
It's one of the medtech's largest partnerships of its kind in North America, as it plans to provide diagnostic imaging and artificial intelligence-enabled applications to the health system over the next decade.
By Greg Slabodkin • June 9, 2020 -
BARDA, Evidation team on early detection of COVID-19 using wearables
As part of an agency program to identify people infected with the virus, the company will analyze sleep and activity data, plus self-reported symptoms, aimed at creating an early warning algorithm.
By Nick Paul Taylor • June 5, 2020 -
Pandemic causes expected wearable shipments to plummet by 27M this year
But analysts expect a drawn-out recovery for the sector as wearables bolster their advanced health monitoring features and consumers remain increasingly health-conscious coming out of the COVID-19 crisis.
By Rebecca Pifer • June 4, 2020 -
Emergency authorization granted to COVID-19 ICU prediction software
The FDA nod for CLEW Medical's system is among a series of regulatory OKs for products designed to inform care for infected patients.
By Nick Paul Taylor • May 28, 2020 -
Sponsored by Alexander Group
How leading commercial organizations will ramp upon re-open
Increased agility helps healthcare commercial leaders adapt to a new buyer journey.
May 27, 2020 -
ResMed, Medtronic embrace remote tech amid COVID-19, say it's here to stay
The crisis has sped adoption of digital health technologies to help hospitals create safe physical distances between healthcare workers and patients. Execs say some could become permanent products.
By Greg Slabodkin • May 26, 2020 -
Philips lung ultrasound, Beckman sepsis diagnostic receive BARDA coronavirus backing
The separate projects aim to incorporate machine learning algorithms to enhance technologies meant to support COVID-19 patients.
By Susan Kelly • May 19, 2020 -
UnitedHealth, Microsoft launch COVID-19 screening app for employers
The healthcare behemoth said it will control employees' medical data and manage opt-in and consent requirements for users. The app will not provide tracking or contact tracing information.
By Rebecca Pifer • May 15, 2020 -
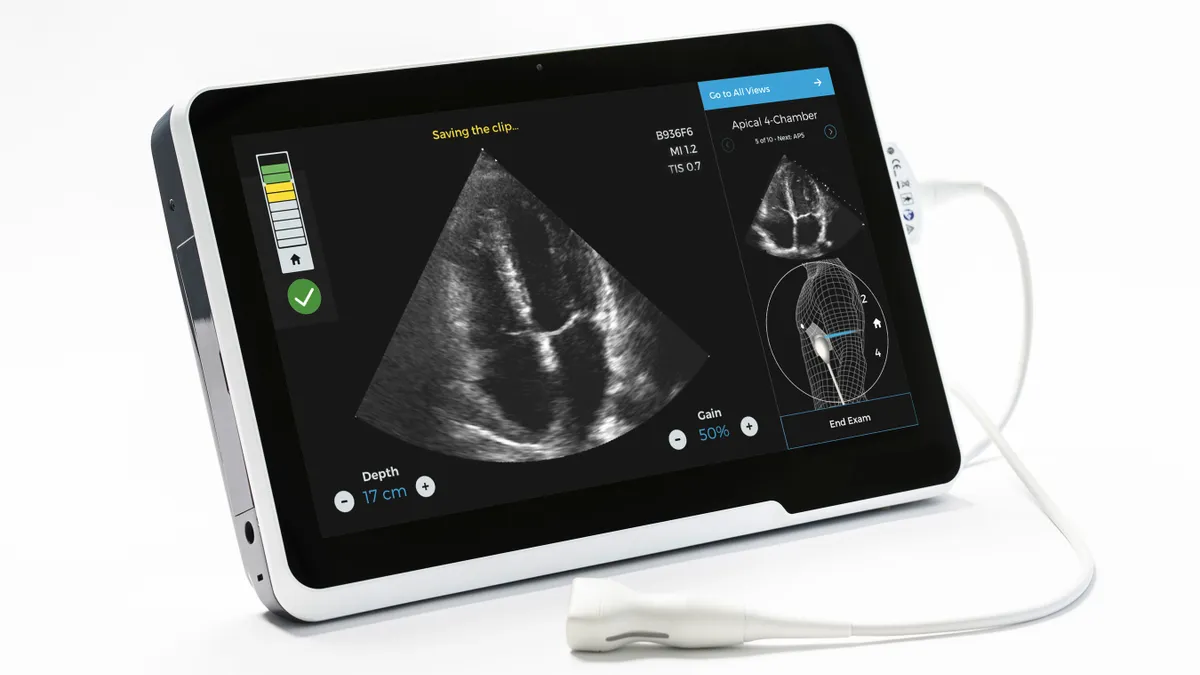
Philips, startups look to deploy new ultrasound tech amid coronavirus
The need for point-of-care imaging for lung and cardiac complications among COVID-19 patients has led to a flurry of expedited FDA reviews.
By Nick Paul Taylor • May 14, 2020 -
Eko algorithm gets FDA nod for cardiac screening of COVID-19 patients
Developed with the Mayo Clinic, Eko's algorithm gained breakthrough device status in December and review was further accelerated given its potential to identify patients at higher risk of poor outcomes with the coronavirus.
By Nick Paul Taylor • May 13, 2020 -
Deep Dive
Telehealth is having a moment. What does its future look like after COVID-19?
Employers should expect workers to embrace telehealth post pandemic, sources told HR Dive, even if concerns about billing and security persist.
By Ryan Golden • May 7, 2020 -
Fitbit launches its 1st large-scale, virtual AFib study
The atrial fibrillation detection effort follows work by wearables rival Apple targeting the stroke risk factor that affects 33.5 million people globally.
By Greg Slabodkin • May 6, 2020 -
COVID-19 spurs 'incredible demand' for ventilators, buoying ResMed's 16% revenue uptick
CEO Mick Farrell told investors sales of breathing assist machines added $35 million in coronavirus-related revenue. However, sleep testing declined by double digits.
By Greg Slabodkin • May 1, 2020 -
FDA encourages remote review of digital pathology slides amid COVID-19
The agency's latest effort to support greater access to medical devices during the pandemic follows a move by CMS that opened the door for pathologists to work away from the lab.
By Susan Kelly • April 27, 2020 -
Digital therapeutic makers see opportunity as pandemic prompts FDA to ease rules
Akili Interactive Labs is rolling out its video game treatment for ADHD for the first time, while Pear Therapeutics weighs how products still in its pipeline may apply during the coronavirus crisis.
By Maria Rachal • April 24, 2020