Digital Health
-
FDA seeks feedback on monitoring real-world performance of AI devices
The agency is looking for ways to detect, assess and mitigate changes to the performance of AI-enabled devices over time.
By Nick Paul Taylor • Oct. 1, 2025 -
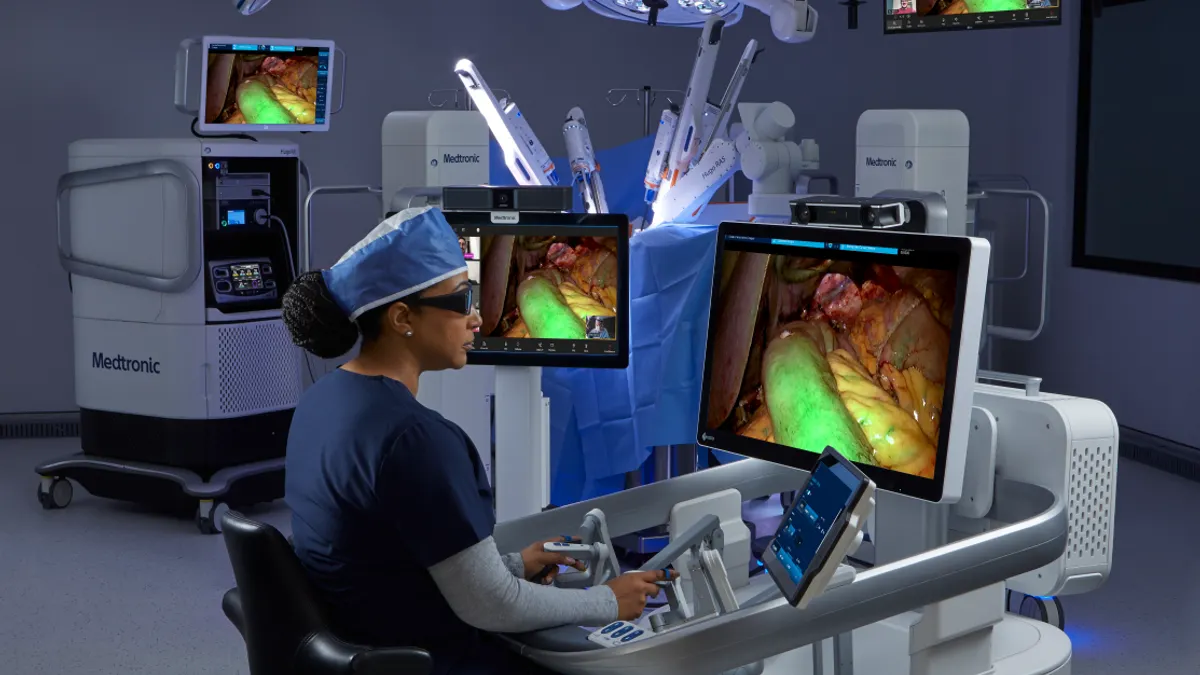
Medtronic expands AI, robotics hub in London; Virtuoso robot gets FDA breakthrough status
The Medtronic center will facilitate surgeon collaboration and AI-powered decision support. At Virtuoso Surgical, a robotic approach to bladder lesion removal is expected to improve precision.
By Susan Kelly • Sept. 24, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Sara Silbiger via Getty Images
Sara Silbiger via Getty Images Trendline
TrendlineTop 5 stories from MedTech Dive
From haphazard layoffs at the Food and Drug Administration to the industry’s current IPO environment and tracking FDA-authorized AI devices, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
Cardiosense names Eric Meizlish as CEO
Cardiosense’s switch to a leader with experience scaling healthtech companies comes as it works to build on the recent 510(k) clearance of CardioTag.
By Nick Paul Taylor • Updated Sept. 25, 2025 -
FDA warns on unauthorized blood pressure, infant monitoring devices
Many of these devices currently sold over-the-counter do not have marketing authorization, the agency said in a notice.
By Elise Reuter • Updated Sept. 18, 2025 -
GOP bill extends telehealth flexibilities, sidesteps ACA subsidies
The continuing resolution would fund the government, and preserve the flexibilities, through Nov. 21. But the bill makes no mention of extending more generous financial assistance for people who buy plans on the exchanges.
By Emily Olsen • Sept. 17, 2025 -
Deep Dive
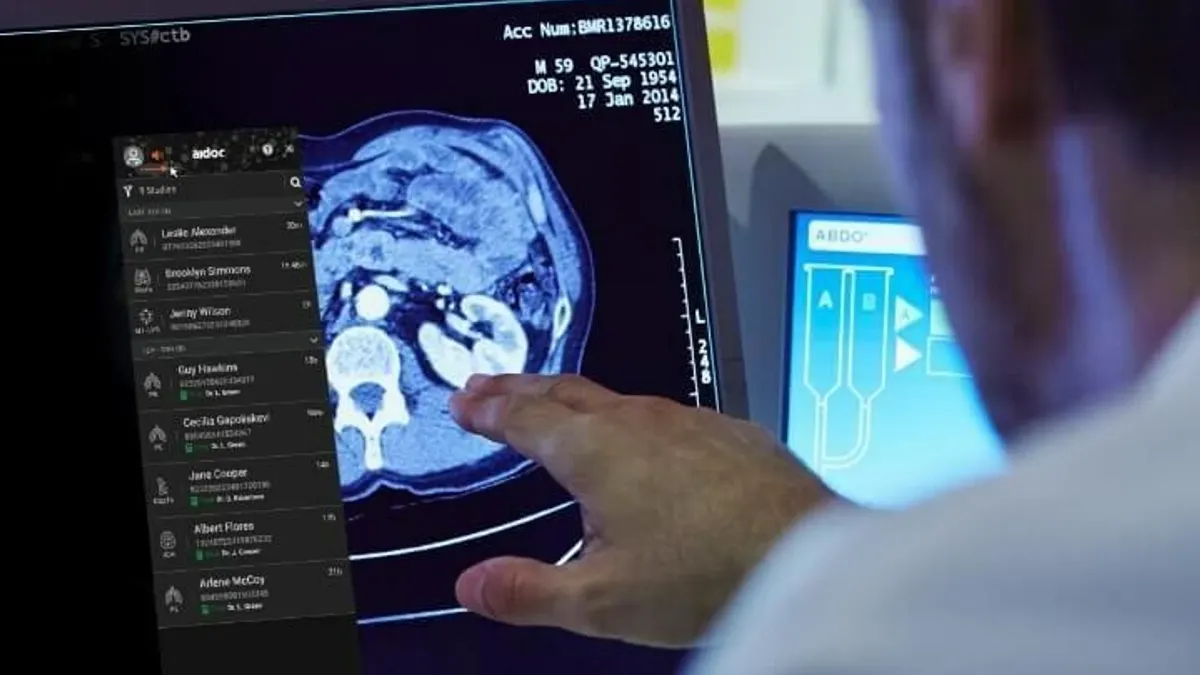
How three companies are using foundation models in radiology
Some firms, such as Aidoc, are working directly with the FDA. Others, such as HOPPR, are offering their foundation models for medtech companies to build their own AI tools.
By Elise Reuter • Sept. 16, 2025 -
Deep Dive
Foundation models are medtech’s newest trend. But what are they?
The AI models, which are built on massive datasets and can be adapted to multiple tasks, have become a part of medtech companies’ lexicon in the past few years.
By Elise Reuter • Sept. 15, 2025 -
AI firm Ketryx raises $39M, adds former Medtronic CEO as investor
The company, which helps medtech companies with AI compliance, has raised more than $55 million to date.
By Elise Reuter • Sept. 5, 2025 -
Deep Dive
White House data sharing plan boasts big ambitions, but has scant details
Improving health data exchange is a worthy goal, but the initiative has to overcome challenges like data security, under-resourced providers and slow technology uptake, experts say.
By Emily Olsen • Aug. 27, 2025 -
Tempus inks $81M Paige buyout to support AI model development
CEO Eric Lefkofsky said that buying Paige “substantially accelerates” Tempus’ effort to build the largest foundation model in oncology.
By Nick Paul Taylor • Aug. 26, 2025 -
Apple updates smart watches with blood oxygen feature after customs ruling
Apple said it was able to bring a redesigned blood oxygen feature to its devices after a recent U.S. customs ruling.
By Elise Reuter • Aug. 14, 2025 -
Q&A
Shan Jegatheeswaran shares J&J’s vision for AI in the OR
By combining surgical video with other insights, Jegatheeswaran hopes Johnson & Johnson can support better outcomes in procedures.
By Elise Reuter • Aug. 13, 2025 -
Trump administration launches health data sharing initiative
More than 60 companies, including Amazon, Google, UnitedHealth and Epic, have pledged to participate in the initiative, which the CMS said would “deliver results” early next year.
By Emily Olsen • July 31, 2025 -
Cardiosense wins FDA clearance for wearable heart monitor
The device captures various types of heart data that could be used in AI models for cardiovascular parameters with the aim of lowering barriers to monitoring.
By Nick Paul Taylor • July 31, 2025 -
Aidoc raises $150M for AI foundation model
The technology, called CARE, is expected to allow for faster development of AI models that can cover more health conditions.
By Elise Reuter • July 24, 2025 -
Whoop’s FDA warning letter sparks debate over blood pressure as a wellness metric
Whoop pushed back on the warning letter, claiming its blood pressure feature for a wearable wristband is for “wellness” and should not be regulated as a medical device.
By Elise Reuter • July 18, 2025 -
Samsung to acquire digital health firm Xealth
The purchase of the Providence spinoff bolsters Samsung’s push to unify health data, including through wearables like the electronics giant’s Galaxy Watch line.
By Emily Olsen • July 10, 2025 -
Q&A
Nvidia’s David Niewolny on the future of AI in medical devices
Nvidia, which is already working with J&J and Medtronic, plans to partner with medtech companies to build platforms for AI-enabled robotics, imaging and other features.
By Elise Reuter • June 2, 2025 -
Sponsored by COMSOL, Inc.
Simulation app introduces personalized oncology care
See one company's idea for bringing personalization and precision to oncology care
By Dixita Patel • May 19, 2025 -
FDA, aiming to speed scientific reviews, names chief AI officer
Commissioner Martin Makary also said the agency would roll out a generative AI system across its centers that will help FDA scientists spend less time on repetitive tasks.
By Susan Kelly • May 9, 2025 -
Abbott integrates diabetes data into Epic’s electronic health records
Linking the systems will allow clinicians to view glucose data captured by Libre glucose sensors within Epic’s systems.
By Nick Paul Taylor • May 1, 2025 -
Senseonics, Sequel partner to use 1-year CGM in automated insulin dosing system
The partnership extends the compatibility of Sequel’s Twiist system to allow people with Type 1 diabetes to pair the device with Senseonics’ Eversense 365 CGM.
By Nick Paul Taylor • April 30, 2025 -
Kandu Health merges with BCI company Neurolutions
The merger brings together Neurolutions’ brain computer interface technology and Kandu Health’s telehealth services to try to improve stroke care.
By Nick Paul Taylor • April 9, 2025 -
Eargo, Hearx merge into one OTC hearing aid firm, receive $100M boost
Hearx, the maker of Lexie hearing aids, and Eargo competed for the OTC hearing aid market created by the FDA in 2022.
By Nick Paul Taylor • April 3, 2025 -
Sponsored by Veeva MedTech
Cardinal Health turns complaints into quality improvements
Transform complaint management with a single source of truth.
March 31, 2025